Cyclooxygenase
From Proteopedia
(Difference between revisions)
| (69 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | '''COX-2''' | + | <StructureSection load='5cox' size='350' side='right' caption='Glycosylated mouse COX-2 tetramer with heme group (PDB code [[5cox]])' > |
| + | == General function == | ||
| + | |||
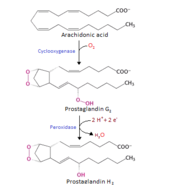
| + | '''Cyclooxygenase COX-1''' and '''COX-2''', also called '''Prostaglandin H2 synthase PGHS-1''', '''Prostaglandin G/H synthase''' and '''PGHS-2''', regulate a key step in prostaglandin and thromboxane synthesis and are the targets of nonsteroidal antiinflammatory drugs (NSAIDs) <ref name="Smith&Langenbach2001">PMID: 11413152</ref> <ref name="Chandrasekharan2002">PMID: 12242329</ref> <ref name="Ghosh2010">PMID: 20508278</ref>. Prostaglandins are implicated in various pathophysiological processes such as inflammatory reactions, gastrointestinal cytoprotection, hemostasis and thrombosis, as well as renal hemodynamics <ref name="Smith&Langenbach2001" /> <ref name="Ghosh2010"/> <ref name="Smith2000">PMID: 10966456</ref>. Whereas COX-1 presents a widespread constitutive expression, COX-2 is undetectable in most normal tissues (except for the central nervous system, kidneys, and seminal vesicles), but is induced by various inflammatory and mitogenic stimuli <ref name="Smith2000" /> <ref name="Ghosh2010"/> <ref name="Rang&Dale2008">Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. UK. 844 p.</ref>. More recently, a third isoform named COX-3 was identified as a COX-1 splicing variant. This new isoform may play a role in processes such as fever and pain <ref name="Ghosh2010"/> <ref name="Chandrasekharan2002"/>. | ||
| - | COX-1 and COX-2, also called PGHS-1 and PGHS-2, regulate a key step in prostaglandin and thromboxane synthesis and are the targets of nonsteroidal antiinflammatory drugs (NSAIDs) <ref name="Smith&Langenbach2001">PMID: 11413152</ref> <ref name="Chandrasekharan2002">PMID: 12242329</ref> <ref name="Ghosh2010">PMID: 20508278</ref>. Prostaglandins are implicated in various pathophysiological processes such as inflammatory reactions, gastrointestinal cytoprotection, hemostasis and thrombosis, as well as renal hemodynamics <ref name="Smith&Langenbach2001" /> <ref name="Ghosh2010"/> <ref name="Smith2000">PMID: 10966456</ref>. Whereas COX-1 presents a widespread constitutive expression, COX-2 is undetectable in most normal tissues (except for the central nervous system, kidneys, and seminal vesicles), but is induced by various inflammatory and mitogenic stimuli <ref name="Smith2000" /> <ref name="Ghosh2010"/> <ref name="Rang&Dale2008">Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. UK. 844 p.</ref>. More recently, a third isoform named COX-3 was identified as a COX-1 splicing variant. This new isoform may play a role in processes such as fever and pain <ref name="Ghosh2010"/> <ref name="Chandrasekharan2002"/>. | ||
Additionally, a high level of COX-2 expression is found usually in cancer cells <ref name="Ghosh2010"/>. For example, COX-2 overexpression is related to poor prognosis in certain breast cancers <ref name="Barnes2007">PMID: 17285134</ref> <ref name="Boland2004">PMID: 14735188</ref> and endometrial adenocarcinomas <ref name="Sales2008">PMID: 18316157</ref>. | Additionally, a high level of COX-2 expression is found usually in cancer cells <ref name="Ghosh2010"/>. For example, COX-2 overexpression is related to poor prognosis in certain breast cancers <ref name="Barnes2007">PMID: 17285134</ref> <ref name="Boland2004">PMID: 14735188</ref> and endometrial adenocarcinomas <ref name="Sales2008">PMID: 18316157</ref>. | ||
| - | + | See also [[Inflammation & Rheumatoid Arthritis]]. | |
| - | [[Image:Reaccion cox.png|thumb|alt=COX reaction|COX reaction]] | + | |
| - | COX-2, unlike COX-1, is induced in inflammatory cells when they are activated by various inflammatory and mitogenic stimuli <ref name="Rang&Dale2008"/> in order to produce the prostanoid mediators of the inflammation. Constitutive levels of COX-2 are generally low in most tissues, although there are some significant exceptions. For example, there is a considerable pool of “constitutive” COX-2 present in the central nervous system (CNS) and some other tissues, although its function is not yet completely clear <ref name="Ghosh2010"/>. | + | ==Patho-physiological Function== |
| + | [[Image:Reaccion cox.png|thumb|left|alt=COX reaction|COX reaction]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | COX-2, unlike COX-1, is induced in inflammatory cells when they are activated by various inflammatory and mitogenic stimuli <ref name="Rang&Dale2008"/> in order to produce the prostanoid mediators that trigger important [[Inflammation|inflammatory processes]] including physiological and pathological situations. Although inflammation is initially a necessary process to fight infection or build up an efficacious inmmune response, when it is maintained or remains uncontrolled it can provoke chronic pathologies and tissue damage. This is why the inhibitios of COX proteins have created considerable interest as potentially potent anti-inflammatory targets what has led to the development of the "coxibs". However, COX-2-produced prostanoids also regulate many important physiological functions such as vascular, bronchial, or gastrointestinal contractility by regulating smooth muscle tone, uterine contractility during labor, and the activity of hormones and fat metabolism among other functions. | ||
| + | |||
| + | This fact makes it difficult to design pure anti-inflamatory drugs based on COX inhibition in the absence of side effects. For instance, both COX-1 and COX-2 help to convert essential fatty acids to other prostanoids that actually reduce [[inflammation]] or serve other regulatory functions. Also, the excess arachidonate that cannot be converted into prostaglandins upon COX inhibition can be derived into leukotriene synthesis thus sustaining allergic reactions, or alternatively into thromboxanes, which may be responsible for the increased clotting and subsequent heart attacks detected with the use of some COX-2 inhibitors ([[Celecoxib]], rofecoxib, ...). | ||
| + | |||
| + | Constitutive levels of COX-2 are generally low in most tissues, although there are some significant exceptions. For example, there is a considerable pool of “constitutive” COX-2 present in the central nervous system (CNS) and some other tissues, although its function is not yet completely clear <ref name="Ghosh2010"/>. | ||
Moreover, COX-1, that is present ubiquitously, has a “housekeeping” role in the body, being involved in tissue homeostasis, and it appears to be responsible for the production of the prostaglandins involved in gastric cytoprotection, platelet aggregation, renal blood flow autoregulation and the initiation of parturition <ref name="Ghosh2010"/>. | Moreover, COX-1, that is present ubiquitously, has a “housekeeping” role in the body, being involved in tissue homeostasis, and it appears to be responsible for the production of the prostaglandins involved in gastric cytoprotection, platelet aggregation, renal blood flow autoregulation and the initiation of parturition <ref name="Ghosh2010"/>. | ||
| + | |||
| + | See [[Aspirin effects on COX aka PGHS]]. | ||
==Structure <ref name="Garavito2003>PMID: 17851687</ref> <ref name="Smith2000"/>== | ==Structure <ref name="Garavito2003>PMID: 17851687</ref> <ref name="Smith2000"/>== | ||
| - | + | ||
| - | In 1994, Picot et al | + | In 1994, Picot ''et al'' published the first three-dimensional (3D) structure of a COX enzyme, the ovine COX-1 complexed with the NSAID flurbiprofen. Soon afterward, the crystal structures of human and murine COX-2 followed. First, the three-dimensional structure of human COX-2 was assessed by means of sequence homology modeling, but in 1996, Luong, C. ''et al'' <ref name="Luong1996">PMID: 8901870</ref> and Kurumbail, R.G ''et al'' <ref name="Kurumbail1996">PMID: 8967954</ref> published two crystal structures of the recombinant human and mouse COX-2 isozymes, respectively, complexed with different selective inhibitors. Given its pharmacological importance as a therapeutic target, drug interactions with COX were one of the first issues to be addressed, and complexes containing a number of different NSAIDs have been studied crystallographically. The structural analysis of COX complexed with substrates or products was more difficult to pursue for a number of technical reasons. However, within the past years, crystal structures of murine COX-2 complexed with AA and EPA have also been determined. |
PGHSs are bifunctional <scene name='SandboxUAM/Mynewscene/22'>homodimers</scene>. Both COX-1 and COX-2 are membrane-bound enzymes and are present on the luminal surfaces of the endoplasmic reticulum and of the inner and outer membranes of the nuclear envelope. However, recently, using cultured endothelial cells and fibroblasts a fraction of COX-2 protein was shown to be localized to plasma membrane in caveolae-like structures <ref name="Perrone2007">PMID: 10551860</ref>. The primary structure of nascent COX-2 is of 604 amino acids and it is processed into a mature form by removal of signal peptides giving a protein of 587 amino acids. PGHS-2 is variably glycosylated at two to four sites, leading to the formation of doublets or sometimes triplets that can be detected on SDS-PAGE. In murine PGHS-2 <scene name='SandboxUAM/Mynewscene/8'>carbohydrate moieties</scene> are linked to Asn-68, Asn-144, and Asn-410 in each monomer <ref name="Vecchio2010">PMID: 20463020</ref>. | PGHSs are bifunctional <scene name='SandboxUAM/Mynewscene/22'>homodimers</scene>. Both COX-1 and COX-2 are membrane-bound enzymes and are present on the luminal surfaces of the endoplasmic reticulum and of the inner and outer membranes of the nuclear envelope. However, recently, using cultured endothelial cells and fibroblasts a fraction of COX-2 protein was shown to be localized to plasma membrane in caveolae-like structures <ref name="Perrone2007">PMID: 10551860</ref>. The primary structure of nascent COX-2 is of 604 amino acids and it is processed into a mature form by removal of signal peptides giving a protein of 587 amino acids. PGHS-2 is variably glycosylated at two to four sites, leading to the formation of doublets or sometimes triplets that can be detected on SDS-PAGE. In murine PGHS-2 <scene name='SandboxUAM/Mynewscene/8'>carbohydrate moieties</scene> are linked to Asn-68, Asn-144, and Asn-410 in each monomer <ref name="Vecchio2010">PMID: 20463020</ref>. | ||
The COX monomer consists of <scene name='SandboxUAM/Mynewscene/12'>three structural domains</scene>: the N-terminal EGF domain, a membrane binding domain (MBD) and a large C-terminal globular catalytic domain containing the heme binding site. The C-terminal segments beyond Pro583 (35 amino acids in COX-2) have not been resolved crystallographically. Collectively, these domains are made up of 25 <scene name='SandboxUAM/Mynewscene/23'>alpha helices</scene>, seven 3<sub>10</sub> <scene name='SandboxUAM/Mynewscene/20'>helices</scene>, four <scene name='SandboxUAM/Mynewscene/24'>beta sheets</scene> as well as five disulfide bonds which contribute to the interface binding of the two individual monomers to complete the enzyme. | The COX monomer consists of <scene name='SandboxUAM/Mynewscene/12'>three structural domains</scene>: the N-terminal EGF domain, a membrane binding domain (MBD) and a large C-terminal globular catalytic domain containing the heme binding site. The C-terminal segments beyond Pro583 (35 amino acids in COX-2) have not been resolved crystallographically. Collectively, these domains are made up of 25 <scene name='SandboxUAM/Mynewscene/23'>alpha helices</scene>, seven 3<sub>10</sub> <scene name='SandboxUAM/Mynewscene/20'>helices</scene>, four <scene name='SandboxUAM/Mynewscene/24'>beta sheets</scene> as well as five disulfide bonds which contribute to the interface binding of the two individual monomers to complete the enzyme. | ||
| - | |||
===Protein domains=== | ===Protein domains=== | ||
| Line 40: | Line 69: | ||
=====Cyclooxygenase Active Site Structure===== | =====Cyclooxygenase Active Site Structure===== | ||
| - | + | PGHS-1 and 2 monomers each contain a 25-°A hydrophobic channel that originates at the membrane binding domain and extends into the core of the globular domain. The MBD forms the entrance and the first half of the channel and allows arachidonate and O2 to enter directly from the apolar compartment of the lipid bilayer. Several amino acids composing the upper half of the channel are uniquely important to cyclooxygenase catalysis. Twenty-four residues line the hydrophobic <scene name='SandboxUAM/Mynewscene/25'>cyclooxygenase active site</scene> with only one difference between the isozymes—Ile at position 523 in PGHS-1 and Val at position 523 in PGHS-2. Amino acids lining the hydrophobic cyclooxygenase active site channel include Leu117, Arg120, Phe205, Phe209, Val344, Ile345, Tyr348, Val349, Leu352, Ser353, Tyr355, Leu359, Phe381, Leu384, Tyr385, Trp387, Phe518, Ile/Val523, Gly526, Ala527, Ser530, Leu531, Gly533, Leu534. <scene name='SandboxUAM/Mynewscene/18'>Only three of the channel residues</scene> are polar (Arg120, Ser353, and Ser530).<scene name='SandboxUAM/Mynewscene/26'>Tyr 385</scene> in its radical form is the responsible for abstracting a proton from arachidonic acid during its conversion to PGG2.<scene name='SandboxUAM/Mynewscene/27'>Ser530</scene> is the site of acetylation by [[Aspirin]] (see [[Aspirin effects on COX aka PGHS|aspirin]]) and <scene name='SandboxUAM/Mynewscene/30'>Arg120</scene>, which is positioned about midway between the entrance and the apex of the active site <ref name="Garavito&DeWitt1999">PMID: 10570255</ref>, binds to the carboxylate groups of fatty acids and many NSAIDs. <br/> | |
| - | PGHS-1 and 2 monomers each contain a 25-°A hydrophobic channel that originates at the membrane binding domain and extends into the core of the globular domain. The MBD forms the entrance and the first half of the channel and allows arachidonate and O2 to enter directly from the apolar compartment of the lipid bilayer. Several amino acids composing the upper half of the channel are uniquely important to cyclooxygenase catalysis. Twenty-four residues line the hydrophobic <scene name='SandboxUAM/Mynewscene/25'>cyclooxygenase active site</scene> with only one difference between the isozymes—Ile at position 523 in PGHS-1 and Val at position 523 in PGHS-2. Amino acids lining the hydrophobic cyclooxygenase active site channel include Leu117, Arg120, Phe205, Phe209, Val344, Ile345, Tyr348, Val349, Leu352, Ser353, Tyr355, Leu359, Phe381, Leu384, Tyr385, Trp387, Phe518, Ile/Val523, Gly526, Ala527, Ser530, Leu531, Gly533, Leu534. <scene name='SandboxUAM/Mynewscene/18'>Only three of the channel residues</scene> are polar (Arg120, Ser353, and Ser530).<scene name='SandboxUAM/Mynewscene/26'>Tyr 385</scene> in its radical form is the responsible for abstracting a proton from arachidonic acid during its conversion to PGG2.<scene name='SandboxUAM/Mynewscene/27'>Ser530</scene> is the site of acetylation by aspirin and <scene name='SandboxUAM/Mynewscene/30'>Arg120</scene>, which is positioned about midway between the entrance and the apex of the active site <ref name="Garavito&DeWitt1999">PMID: 10570255</ref>, binds to the carboxylate groups of fatty acids and many NSAIDs. <br/> | + | |
| - | + | ||
| - | + | ||
==NSAIDs== | ==NSAIDs== | ||
| - | + | Non-steroid anti-inflammatory [[Pharma drugs|drugs]] are a chemically heterogeneous group of compounds whose major function is the inhibition of cyclooxygenases (Table 1). Apart from their anti-inflammatory effect, they also present analgesic and antipyretic properties <ref name="Rang&Dale2008"/>. | |
| - | + | ||
| - | + | ||
| - | Non-steroid anti-inflammatory drugs are a chemically heterogeneous group of compounds whose major function is the inhibition of cyclooxygenases (Table 1). Apart from their anti-inflammatory effect, they also present analgesic and antipyretic properties <ref name="Rang&Dale2008"/>. | + | |
Classical NSAIDs, as salicylate or phenoprofen, are mostly inhibitors of both isoenzymes, although each isoform is inhibited in a different level (Table 2). Chronic users of NSAIDs develop gastric ulcers or gastrointestinal complications, explained by the inhibition of COX-1. For this reason, selective inhibitors of COX-2, as <scene name='SandboxUAM/Mynewscene/33'>celecoxib</scene>, valdecoxib and etoricoxib, have been developed <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. They don’t cause gastric pathology, but they has been proven to be responsible of nephrotoxicity in some patients. | Classical NSAIDs, as salicylate or phenoprofen, are mostly inhibitors of both isoenzymes, although each isoform is inhibited in a different level (Table 2). Chronic users of NSAIDs develop gastric ulcers or gastrointestinal complications, explained by the inhibition of COX-1. For this reason, selective inhibitors of COX-2, as <scene name='SandboxUAM/Mynewscene/33'>celecoxib</scene>, valdecoxib and etoricoxib, have been developed <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/>. They don’t cause gastric pathology, but they has been proven to be responsible of nephrotoxicity in some patients. | ||
| Line 57: | Line 80: | ||
The inhibition mechanism consists of the entrance of the drug by the hydrophobic channel and the formation of hydrogen bonds with Arg120. This interaction prevents the fatty acids from entering the catalytic site. Selectivity of COX-2 inhibitors is mainly mediated by the substitution of Ile523 in COX-1 with Val523 in COX-2, which results in the presence of a small side pocket adjacent to the active site channel, appreciably increasing the volume of the COX-2 active site <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/> <ref name="Smith2000"/>. The effect of this change is compounded by the substitution of Val434 in COX-2 for Ile434 in COX-1 within the second group of amino acids conforming the active site <ref name="Garavito&DeWitt1999"/>. The combination of these two substitutions in COX-2 allows a neighboring amino acid, Phe518, to swing out of the way, which further increases access to the side pocket <ref name="Garavito&DeWitt1999"/>. | The inhibition mechanism consists of the entrance of the drug by the hydrophobic channel and the formation of hydrogen bonds with Arg120. This interaction prevents the fatty acids from entering the catalytic site. Selectivity of COX-2 inhibitors is mainly mediated by the substitution of Ile523 in COX-1 with Val523 in COX-2, which results in the presence of a small side pocket adjacent to the active site channel, appreciably increasing the volume of the COX-2 active site <ref name="Ghosh2010"/> <ref name="Rang&Dale2008"/> <ref name="Smith2000"/>. The effect of this change is compounded by the substitution of Val434 in COX-2 for Ile434 in COX-1 within the second group of amino acids conforming the active site <ref name="Garavito&DeWitt1999"/>. The combination of these two substitutions in COX-2 allows a neighboring amino acid, Phe518, to swing out of the way, which further increases access to the side pocket <ref name="Garavito&DeWitt1999"/>. | ||
| - | + | <br/> | |
| + | <br/> | ||
{| class="wikitable" align="left" | {| class="wikitable" align="left" | ||
| Line 66: | Line 90: | ||
|- | |- | ||
| rowspan="2" | Propionic | | rowspan="2" | Propionic | ||
| - | | Naproxen | + | | [[Naproxen]] |
|- | |- | ||
| - | | Ibuprofen | + | | [[Ibuprofen]] |
|- | |- | ||
| - | | Para-aminophenols || Paracetamol | + | | Para-aminophenols || [[Paracetamol]] |
|- | |- | ||
| Indolacetic || Indometacin | | Indolacetic || Indometacin | ||
| Line 76: | Line 100: | ||
| Pirrolacetic || Ketorolac | | Pirrolacetic || Ketorolac | ||
|- | |- | ||
| - | | Phenilacetic || Diclofenac | + | | Phenilacetic || [[Diclofenac]] |
|- | |- | ||
| Piranoidacetic || Etodolac | | Piranoidacetic || Etodolac | ||
| Line 96: | Line 120: | ||
| <center> 100-1000 </center> | | <center> 100-1000 </center> | ||
|- | |- | ||
| - | | Naproxen | + | | [[Naproxen]] |
| <center> 1-10 </center> | | <center> 1-10 </center> | ||
|- | |- | ||
| Line 108: | Line 132: | ||
| <center> 1 </center> | | <center> 1 </center> | ||
|- | |- | ||
| - | | Diclofenac | + | | [[Diclofenac]] |
| <center> 1-0.1 </center> | | <center> 1-0.1 </center> | ||
|- | |- | ||
| Line 118: | Line 142: | ||
|+ Table 2: Selectivity of some NSAIDs (adapted from <ref name="Rang&Dale2008"/>) | |+ Table 2: Selectivity of some NSAIDs (adapted from <ref name="Rang&Dale2008"/>) | ||
|} | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| Line 137: | Line 191: | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | See also<br /> | ||
| + | [[NSAID Pharmacokinetics]]<br /> | ||
| + | [[Cyclooxygenase Inhibitor Pharmacokinetics]]<br /> | ||
| + | [[Treatments:Inflammation]]<br /> | ||
| + | [[Treatments:NSAID References]]<br /> | ||
| + | [[Flurbiprofen]]. | ||
==Regulation== | ==Regulation== | ||
| Line 169: | Line 240: | ||
The human COX-2 3’-UTR has several polyadenylation sites. COX-2 uses two alternative polyadenylation sites, in a tissue-specific manner, which derives in the formation of 2 COX-2 mRNAs: one with 2.8 kb and another one with 4.6 kb <ref name="Hall-Pogar2005">PMID: 15872218</ref>. It is known that selection of the proximal polyadenylation signal is enhanced by presence of additional USEs (Upstream Sequence Elements) where four RNA-binding proteins (U1A, PTB, p54nrb and PSF) can bind, enhancing the recruitment and stabilization of core adenylation factors on the COX-2 mRNA <ref name="Hall-Pogar2007">PMID: 17507659</ref>. | The human COX-2 3’-UTR has several polyadenylation sites. COX-2 uses two alternative polyadenylation sites, in a tissue-specific manner, which derives in the formation of 2 COX-2 mRNAs: one with 2.8 kb and another one with 4.6 kb <ref name="Hall-Pogar2005">PMID: 15872218</ref>. It is known that selection of the proximal polyadenylation signal is enhanced by presence of additional USEs (Upstream Sequence Elements) where four RNA-binding proteins (U1A, PTB, p54nrb and PSF) can bind, enhancing the recruitment and stabilization of core adenylation factors on the COX-2 mRNA <ref name="Hall-Pogar2007">PMID: 17507659</ref>. | ||
| + | |||
| + | ==Additional Resources== | ||
| + | * For more information about inflammation, see: [[Inflammation]] | ||
| + | * For information about other Pharmaceutical Therapeutics, see: [[Pharmaceutical Therapeutics]] | ||
| + | * [[UMass Chem 423 Student Projects 2011-1#Cyclooxygenase|UMass Chem 423 Student Projects 2011-1 Cyclooxygenase]] | ||
| + | *[[Aspirin effects on COX aka PGHS]] | ||
| + | |||
| + | == 3D Structures of cyclooxygenase == | ||
| + | [[Cyclooxygenase 3D structures]] | ||
| + | |||
| + | </StructureSection> | ||
| + | |||
| + | =='''Authorship'''== | ||
| + | |||
| + | This page has been constructed and edited by '''Maria Saiz, Rafael Gonzalez & Eva Garcia''', students of the Biomedicine Master of the Universidad Autonoma de Madrid (Spain) under the supervision of Dr. Cristina Murga, as a contribution to the Molecular Pharmacology Course of the doctoral programme in Biosciences. | ||
| - | == | + | ==References== |
<references /> | <references /> | ||
[[Category: Enzyme]] | [[Category: Enzyme]] | ||
| - | [[Category: | + | [[Category:Topic Page]] |
Current revision
| |||||||||||
Authorship
This page has been constructed and edited by Maria Saiz, Rafael Gonzalez & Eva Garcia, students of the Biomedicine Master of the Universidad Autonoma de Madrid (Spain) under the supervision of Dr. Cristina Murga, as a contribution to the Molecular Pharmacology Course of the doctoral programme in Biosciences.
References
- ↑ 1.0 1.1 Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001 Jun;107(12):1491-5. PMID:11413152 doi:10.1172/JCI13271
- ↑ 2.0 2.1 Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13926-31. Epub 2002 Sep 19. PMID:12242329 doi:10.1073/pnas.162468699
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep. 2010 Mar-Apr;62(2):233-44. PMID:20508278
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145-82. PMID:10966456 doi:10.1146/annurev.biochem.69.1.145
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 Rang HP, Dale MM, Ritter JM, Flower RJ. 2008. Pharmacology. Elsevier. 6th edition. UK. 844 p.
- ↑ Barnes NL, Warnberg F, Farnie G, White D, Jiang W, Anderson E, Bundred NJ. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007 Feb 26;96(4):575-82. Epub 2007 Feb 6. PMID:17285134 doi:10.1038/sj.bjc.6603593
- ↑ Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004 Jan 26;90(2):423-9. PMID:14735188 doi:10.1038/sj.bjc.6601534
- ↑ Sales KJ, Grant V, Jabbour HN. Prostaglandin E2 and F2alpha activate the FP receptor and up-regulate cyclooxygenase-2 expression via the cyclic AMP response element. Mol Cell Endocrinol. 2008 Mar 26;285(1-2):51-61. Epub 2008 Feb 3. PMID:18316157 doi:10.1016/j.mce.2008.01.016
- ↑ Perrone G, Zagami M, Altomare V, Battista C, Morini S, Rabitti C. COX-2 localization within plasma membrane caveolae-like structures in human lobular intraepithelial neoplasia of the breast. Virchows Arch. 2007 Dec;451(6):1039-45. Epub 2007 Sep 13. PMID:17851687 doi:10.1007/s00428-007-0506-4
- ↑ Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol. 1996 Nov;3(11):927-33. PMID:8901870
- ↑ Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996 Dec 19-26;384(6610):644-8. PMID:8967954 doi:http://dx.doi.org/10.1038/384644a0
- ↑ Spencer AG, Thuresson E, Otto JC, Song I, Smith T, DeWitt DL, Garavito RM, Smith WL. The membrane binding domains of prostaglandin endoperoxide H synthases 1 and 2. Peptide mapping and mutational analysis. J Biol Chem. 1999 Nov 12;274(46):32936-42. PMID:10551860
- ↑ Vecchio AJ, Simmons DM, Malkowski MG. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J Biol Chem. 2010 Jul 16;285(29):22152-63. Epub 2010 May 12. PMID:20463020 doi:10.1074/jbc.M110.119867
- ↑ Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol. 1996 Nov;3(11):927-33. PMID:8901870
- ↑ 15.0 15.1 15.2 Garavito RM, DeWitt DL. The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. Biochim Biophys Acta. 1999 Nov 23;1441(2-3):278-87. PMID:10570255
- ↑ FitzGerald GA, Loll P. COX in a crystal ball: current status and future promise of prostaglandin research. J Clin Invest. 2001 Jun;107(11):1335-7. PMID:11390412 doi:10.1172/JCI13037
- ↑ Harper KA, Tyson-Capper AJ. Complexity of COX-2 gene regulation. Biochem Soc Trans. 2008 Jun;36(Pt 3):543-5. PMID:18482003 doi:10.1042/BST0360543
- ↑ Deng WG, Zhu Y, Wu KK. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation. J Biol Chem. 2003 Feb 14;278(7):4770-7. Epub 2002 Dec 5. PMID:12471036 doi:10.1074/jbc.M209286200
- ↑ Song SH, Jong HS, Choi HH, Inoue H, Tanabe T, Kim NK, Bang YJ. Transcriptional silencing of Cyclooxygenase-2 by hyper-methylation of the 5' CpG island in human gastric carcinoma cells. Cancer Res. 2001 Jun 1;61(11):4628-35. PMID:11389100
- ↑ Tyson-Capper AJ, Cork DM, Wesley E, Shiells EA, Loughney AD. Characterization of cellular retinoid-binding proteins in human myometrium during pregnancy. Mol Hum Reprod. 2006 Nov;12(11):695-701. Epub 2006 Sep 7. PMID:16959971 doi:10.1093/molehr/gal070
- ↑ 21.0 21.1 Yamaguchi K, Lantowski A, Dannenberg AJ, Subbaramaiah K. Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2. J Biol Chem. 2005 Sep 23;280(38):32569-77. Epub 2005 Jul 1. PMID:15994313 doi:10.1074/jbc.M503201200
- ↑ Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3'-untranslated region. J Biol Chem. 2000 Apr 21;275(16):11750-7. PMID:10766797
- ↑ Mukhopadhyay D, Houchen CW, Kennedy S, Dieckgraefe BK, Anant S. Coupled mRNA stabilization and translational silencing of cyclooxygenase-2 by a novel RNA binding protein, CUGBP2. Mol Cell. 2003 Jan;11(1):113-26. PMID:12535526
- ↑ Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ. Regulation of cyclooxgenase-2 mRNA stability by taxanes: evidence for involvement of p38, MAPKAPK-2, and HuR. J Biol Chem. 2003 Sep 26;278(39):37637-47. Epub 2003 Jun 25. PMID:12826679 doi:10.1074/jbc.M301481200
- ↑ Hall-Pogar T, Zhang H, Tian B, Lutz CS. Alternative polyadenylation of cyclooxygenase-2. Nucleic Acids Res. 2005 May 4;33(8):2565-79. Print 2005. PMID:15872218 doi:10.1093/nar/gki544
- ↑ Hall-Pogar T, Liang S, Hague LK, Lutz CS. Specific trans-acting proteins interact with auxiliary RNA polyadenylation elements in the COX-2 3'-UTR. RNA. 2007 Jul;13(7):1103-15. Epub 2007 May 16. PMID:17507659 doi:10.1261/rna.577707
Proteopedia Page Contributors and Editors (what is this?)
Cristina Murga, Michal Harel, David Canner, María Laura Saiz Álvarez, Alexander Berchansky