Nicotinic Acetylcholine Receptor
From Proteopedia
(Difference between revisions)
| (26 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='' size='450' side='right' scene='Journal:JBSD:16/Cv/2' caption='Nicotinic Acetylcholine Receptor, PDB code [[2bg9]]'> | ||
==Nicotinic Acetylcholine Receptor== | ==Nicotinic Acetylcholine Receptor== | ||
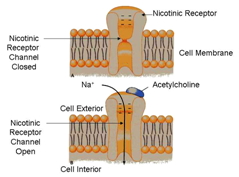
| - | + | The '''nicotinic acetylcholine receptor''' is a key protein in neuronal communication. This protein effectively converts neurotransmitter binding into a membrane depolarization event. The protein combines neurotransmitter binding sites, specifically [[acetylcholine]], with a cationic ion channel, specifically sodium (Na). | |
| - | The nicotinic acetylcholine receptor is a key protein in neuronal communication. This protein effectively converts neurotransmitter binding into a membrane depolarization event. The protein combines neurotransmitter binding sites, specifically [[acetylcholine]], with a cationic ion channel, specifically sodium (Na). | + | [[Image:Nicotinic receptor.jpg|300px]]<br /> |
== Structure == | == Structure == | ||
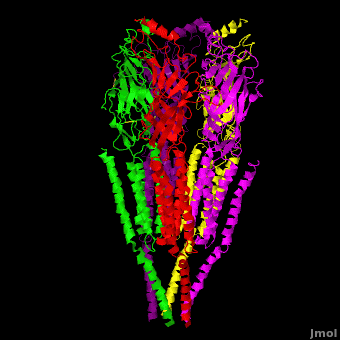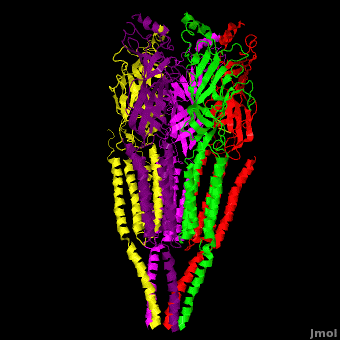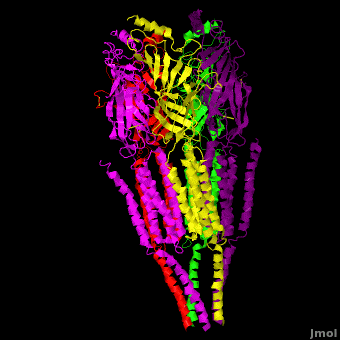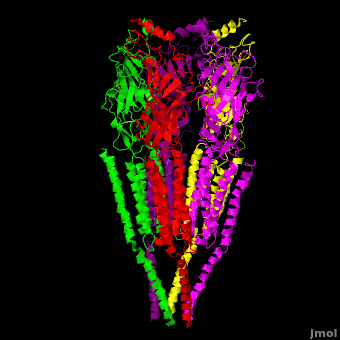
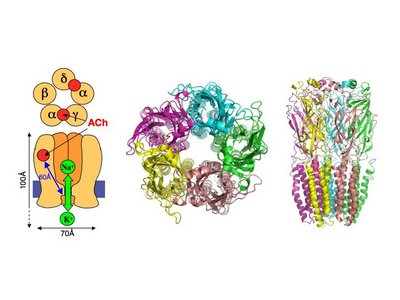
| - | The receptor is a transmembrane pentameric glycoprotein. It has a weight of approximately 300,000 Daltons. It cylindrical in appearance by electron microscopy approximately 16nm in length and 8nm in diameter. The main ion channel is composed of a water pore that runs through the entire length of the protein. If viewed from the synaptic cleft, the protein will look like a pseudo-symmetrical rosette shown in the picture below composed of 10 different | + | The receptor is a transmembrane pentameric glycoprotein. It has a weight of approximately 300,000 Daltons. It cylindrical in appearance by electron microscopy approximately 16nm in length and 8nm in diameter. The main ion channel is composed of a water pore that runs through the entire length of the protein. If viewed from the synaptic cleft, the protein will look like a pseudo-symmetrical rosette shown in the picture below composed of 10 different alpha and 4 different beta subunits. |
| + | *<scene name='58/584302/Cv/1'>Side view</scene>. | ||
| + | *<scene name='58/584302/Cv/2'>View from extracellular side</scene>. | ||
| + | *<scene name='58/584302/Cv/3'>View from cytoplasmic side</scene>. | ||
[[Image:NR1.jpg]] | [[Image:NR1.jpg]] | ||
| Line 12: | Line 16: | ||
This protein carries anywhere from 2 to 5 [[acetylcholine]] binding sites which are located at the interface between two subunits. Each subunit contributes 3 loops to the binding site. There is also a "principle" side and a "complimentary" side of the subunits. The principle side binding nicotine with a high degree of specificity and the complimentary side binding a wide variety of acetylcholine like molecules. | This protein carries anywhere from 2 to 5 [[acetylcholine]] binding sites which are located at the interface between two subunits. Each subunit contributes 3 loops to the binding site. There is also a "principle" side and a "complimentary" side of the subunits. The principle side binding nicotine with a high degree of specificity and the complimentary side binding a wide variety of acetylcholine like molecules. | ||
| + | |||
| + | See also [[Binding site of AChR]] and [[Acetylcholine Receptor and its Reaction to Cobra Venom]]. | ||
=== Ion Channel === | === Ion Channel === | ||
| - | The ion channel of the protein is a 20 membered alpha helix bundle. This channel also contributes to the receptor function in three critical aspects: it contains a gating mechanism, it contains a water pore to | + | The ion channel of the protein is a 20 membered alpha helix bundle. This channel also contributes to the receptor function in three critical aspects: it contains a gating mechanism, it contains a water pore to stabilize ions, and it has a selectivity filter for ion charge. As with most transmembrane alpha helix bundles, it is hydrophobic around the edges to effectively be supported within the membrane, and it is hydrophilic on the inner portion to transport charged ions. |
== Physiology == | == Physiology == | ||
| - | The receptor is a | + | The receptor is a cylindrically-shaped protein. It is embedded in the cell wall of post synaptic nerves at the skeletal neuromuscular junction. The receptor acts as a chemically controlled sodium (Na) channel also known as a [[ligand gated channel]]. |
When in the presence of [[acetylcholine]], the receptor undergoes a conformational change opening up the channel to an influx of sodium (Na) within the cell. When this happens the cell undergoes a depolarization event that triggers an action potential to propagate along the rest of the cell stimulating, for example, a muscle response. | When in the presence of [[acetylcholine]], the receptor undergoes a conformational change opening up the channel to an influx of sodium (Na) within the cell. When this happens the cell undergoes a depolarization event that triggers an action potential to propagate along the rest of the cell stimulating, for example, a muscle response. | ||
| - | The opening of these channels only lasts for a millisecond due to [[cholinesterase]] being present and breaking down [[acetylcholine]] attached to the receptor causing the receptor to close again. Introduction of [[ | + | The opening of these channels only lasts for a millisecond due to [[cholinesterase]] being present and breaking down [[acetylcholine]] attached to the receptor causing the receptor to close again. Introduction of [[Acetylcholinesterase inhibitors]] can cause a depolarization block. It works by creating a prolonged refractory period in the depolarization event promoted by the opening of the receptor channel. |
=== Locations === | === Locations === | ||
| Line 37: | Line 43: | ||
=== Clinical Findings === | === Clinical Findings === | ||
| - | Findings due to nicotinic stimulation from [[ | + | Findings due to nicotinic stimulation from [[Acetylcholinesterase inhibitors]] |
* Neuromuscular junctions of skeletal muscles | * Neuromuscular junctions of skeletal muscles | ||
| - | ** | + | ** Fasciculations and myotonic jerks |
** Weakness and paralysis of the muscle | ** Weakness and paralysis of the muscle | ||
| Line 66: | Line 72: | ||
'''F'''riday '''F'''asciculations | '''F'''riday '''F'''asciculations | ||
| + | See also: | ||
| + | *[[Receptor]] | ||
| + | *[[Transmembrane (cell surface) receptors]] | ||
| + | *[[Ionotropic receptors]] | ||
| + | |||
| + | == 3D Structures of nicotinic acetylcholine receptor == | ||
| + | [[Acetyl choline receptor 3D structures]] | ||
| + | </StructureSection> | ||
== References == | == References == | ||
| Line 74: | Line 88: | ||
3. Adcock C, Smith GR, Sansom MS. The nicotinic acetylcholine receptor: from molecular model to single-channel conductance. Eur Biophys J. 2000;29:29–37. | 3. Adcock C, Smith GR, Sansom MS. The nicotinic acetylcholine receptor: from molecular model to single-channel conductance. Eur Biophys J. 2000;29:29–37. | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
1. Cholinesterase Inhibitors: Including Insecticides and Chemical Warfare Nerve Agents, Agency for Toxiz Substances and Disease Regulation accessed 5/2/14
2. Pierre-Jean Corringer and Jean-Pierre Changeux (2008) Nicotinic acetylcholine receptors. Scholarpedia, 3(1):3468.
3. Adcock C, Smith GR, Sansom MS. The nicotinic acetylcholine receptor: from molecular model to single-channel conductance. Eur Biophys J. 2000;29:29–37.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, Alex Pennington