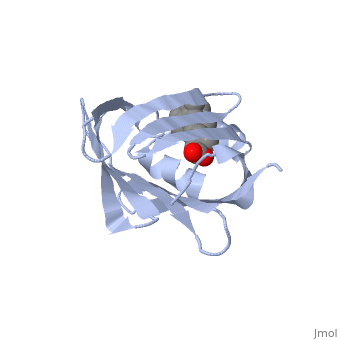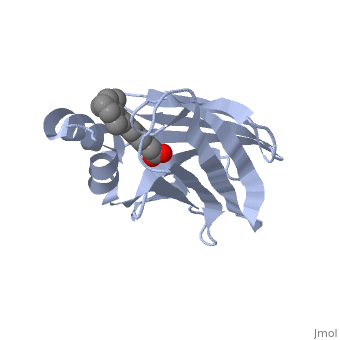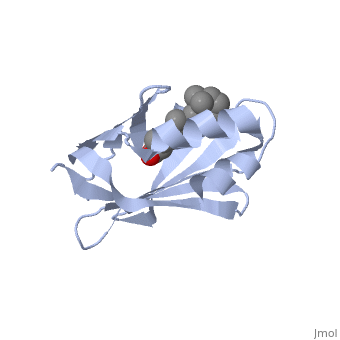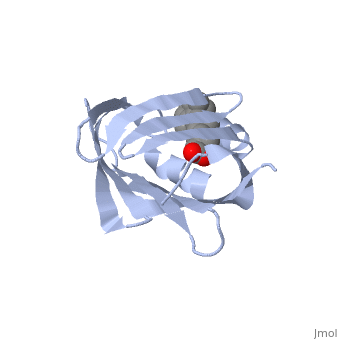
1cbs
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='1cbs' size='340' side='right'caption='[[1cbs]], [[Resolution|resolution]] 1.80Å' scene=''> | <StructureSection load='1cbs' size='340' side='right'caption='[[1cbs]], [[Resolution|resolution]] 1.80Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[1cbs]] is a 1 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[1cbs]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1CBS OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1CBS FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.8Å</td></tr> |
| - | <tr id=' | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=REA:RETINOIC+ACID'>REA</scene></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1cbs FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1cbs OCA], [https://pdbe.org/1cbs PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1cbs RCSB], [https://www.ebi.ac.uk/pdbsum/1cbs PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1cbs ProSAT]</span></td></tr> |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [ | + | [https://www.uniprot.org/uniprot/RABP2_HUMAN RABP2_HUMAN] Transports retinoic acid to the nucleus. Regulates the access of retinoic acid to the nuclear retinoic acid receptors. |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 20: | Line 20: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1cbs ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1cbs ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | BACKGROUND: Retinoic acid (RA) plays a fundamental role in diverse cellular activities. Cellular RA binding proteins (CRABPs) are thought to act by modulating the amount of RA available to nuclear RA receptors. CRABPs and cellular retinol-binding proteins (CRBPs) share a unique fold of two orthogonal beta-sheets that encapsulate their ligands. It has been suggested that a trio of residues are the prime determinants defining the high specificity of CRBPs and CRABPs for their physiological ligands. RESULTS: Bovine/murine CRABP I and human CRABP II have been crystallized in complex with their natural ligand, all-trans-RA. Human CRABP II has also been crystallized in complex with a synthetic retinoid, 'compound 19'. Their structures have been determined and refined at resolutions of 2.9 A, 1.8 A and 2.2 A, respectively. CONCLUSIONS: The retinoid-binding site in CRABPs differs significantly from that observed in CRBP. Structural changes in three juxtaposed areas of the protein create a new, displaced binding site for RA. The carboxylate of the ligand interacts with the expected trio of residues (Arg132, Tyr134 and Arg111; CRABP II numbering). The RA ligand is almost flat with the beta-ionone ring showing a significant deviation (-33 degrees) from a cis conformation relative to the isoprene tail. The edge atoms of the beta-ionone ring are accessible to solvent in a suitable orientation for presentation to metabolizing enzymes. The bulkier synthetic retinoid causes small conformational changes in the protein structure. | ||
| - | |||
| - | Crystal structures of cellular retinoic acid binding proteins I and II in complex with all-trans-retinoic acid and a synthetic retinoid.,Kleywegt GJ, Bergfors T, Senn H, Le Motte P, Gsell B, Shudo K, Jones TA Structure. 1994 Dec 15;2(12):1241-58. PMID:7704533<ref>PMID:7704533</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 1cbs" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
| Line 36: | Line 27: | ||
*[[Gustavo Elberto Epalza Sanchez/Sandbox 1|Gustavo Elberto Epalza Sanchez/Sandbox 1]] | *[[Gustavo Elberto Epalza Sanchez/Sandbox 1|Gustavo Elberto Epalza Sanchez/Sandbox 1]] | ||
*[[Molecular Playground/CRABP I (Cellular Retinoic Acid Binding Protein)|Molecular Playground/CRABP I (Cellular Retinoic Acid Binding Protein)]] | *[[Molecular Playground/CRABP I (Cellular Retinoic Acid Binding Protein)|Molecular Playground/CRABP I (Cellular Retinoic Acid Binding Protein)]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Homo sapiens]] |
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: Bergfors | + | [[Category: Bergfors T]] |
| - | [[Category: Jones | + | [[Category: Jones TA]] |
| - | [[Category: Kleywegt | + | [[Category: Kleywegt GJ]] |
| - | + | ||
Current revision
CRYSTAL STRUCTURE OF CELLULAR RETINOIC-ACID-BINDING PROTEINS I AND II IN COMPLEX WITH ALL-TRANS-RETINOIC ACID AND A SYNTHETIC RETINOID
| |||||||||||