|
|
| (23 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
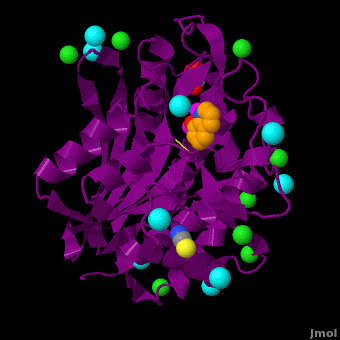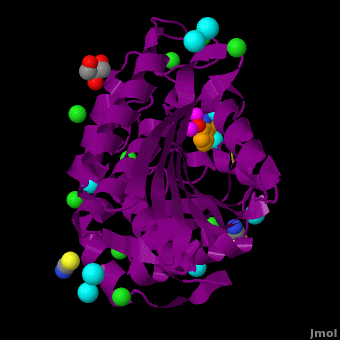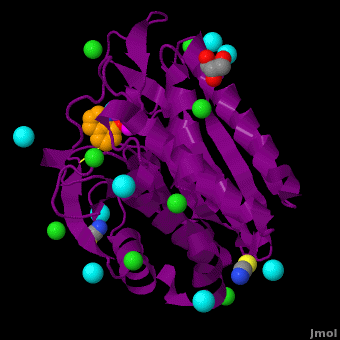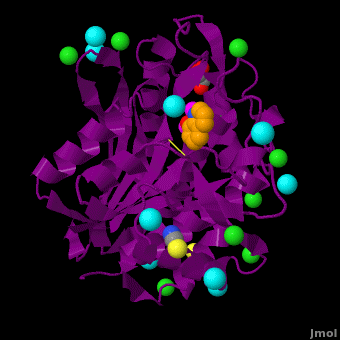
| - | <StructureSection load='VH9.pdb' size='350' side='right' scene='Journal:JBIC:15/Cv/1' caption='Bacterial leucine aminopeptidase complex with 8-hydroxyquinoline, glycerol, SCN, Zn+2 (magenta), Na+ (cyan) and Cl- (green) ions (PDB code [[3vh9]])'>[[Aminopeptidase|Aminopeptidases]] (AP) ([[EC]] 3) are metal - mostly Zn-dependent enzymes involved in the digestion of proteins. Cytosol AP (Cyt-AP), deblocking AP (DAP) and AP N (APN) remove N-terminal amino acids. The AP are classified by the amino acid which they hydrolyze. Other types of AP are:<br /> | + | <StructureSection load='' size='350' side='right' scene='Journal:JBIC:15/Cv/1' caption='Bacterial leucine aminopeptidase complex with 8-hydroxyquinoline, glycerol, SCN, Zn+2 (magenta), Na+ (cyan) and Cl- (green) ions (PDB code [[3vh9]])'> |
| | + | __TOC__ |
| | + | == Function == |
| | + | |
| | + | [[Aminopeptidase|Aminopeptidases]] (AP) ([[EC]] 3) are metal - mostly Zn-dependent enzymes involved in the digestion of proteins. '''Cytosol AP''' (Cyt-AP) and '''AP N''' (APN) remove N-terminal amino acids. The AP are classified by the amino acid which they hydrolyze. Other types of AP are:<br /> |
| | * '''Cold-activated AP''' (Col-AP)<br /> | | * '''Cold-activated AP''' (Col-AP)<br /> |
| | * '''Heat stable AP''' from ''Thermus thermophilus'' (AmpT)<br /> | | * '''Heat stable AP''' from ''Thermus thermophilus'' (AmpT)<br /> |
| | * '''AP from ''Staphylococcus aureus''''' (AmpS)<br /> | | * '''AP from ''Staphylococcus aureus''''' (AmpS)<br /> |
| | * '''SGAP''' from ''Stereomyces griseus''. See details in [[Streptomyces griseus Aminopeptidase (SGAP)]].<br /> | | * '''SGAP''' from ''Stereomyces griseus''. See details in [[Streptomyces griseus Aminopeptidase (SGAP)]].<br /> |
| - | * '''Alanine aminopeptidase''' is called '''aminopeptidase N'''. See details in [[Aminopeptidase N]].<br '> | + | * '''Alanine aminopeptidase''' is called '''aminopeptidase N'''. See details in [[Aminopeptidase N]].<br /> |
| - | Aminopeptidases catalyze a release of an N-terminal amino acid from a peptide, amide, or arylamide. Beta-peptidyl AP (BapA) cleaves N-terminal β-homoamino acid from peptides of length 2 to 6.
| + | * '''Aminopeptidase C''' is a Phe aminopeptidase from ''Aspergillus niger''<ref>PMID:12571053</ref>.<br /> |
| | + | * '''Deblocking aminopeptidase''' (DAP) is an exoprotease aminopeptidase which can release N-terminal amino acids from blocked peptides<ref>PMID:21670507</ref>.<br /> |
| | + | * '''Beta-peptidyl AP''' (BapA) cleaves N-terminal β-homoamino acid from peptides of length 2 to 6.<ref>PMID:8440407</ref><br />. |
| | + | * '''M1 family AP''' are Zn+2 containing amino peptidases<ref>PMID:25530263</ref><br /> |
| | + | Aminopeptidases catalyze a release of an N-terminal amino acid from a peptide, amide, or arylamide. |
| | | | |
| | == Aminopeptidase from ''Aeromonas proteolytica''<ref>DOI 10.1007/s00775-012-0873-4</ref> == | | == Aminopeptidase from ''Aeromonas proteolytica''<ref>DOI 10.1007/s00775-012-0873-4</ref> == |
| Line 18: |
Line 26: |
| | | | |
| | The <scene name='Aminopeptidase/Active_site/1'>active site</scene> of the enzyme contains two Zn2+ ions with His85 and Asp160 as ligands for one ion, and Glu132 and His247 as ligands for the second ion. Asp97 is a common ligand to both ions. What appears to be a phosphate anion is bound to both zinc atoms, replacing the water molecule/hydroxide ion normally found in this class of enzyme. See details of SGAP in [[Streptomyces griseus Aminopeptidase (SGAP)]]. | | The <scene name='Aminopeptidase/Active_site/1'>active site</scene> of the enzyme contains two Zn2+ ions with His85 and Asp160 as ligands for one ion, and Glu132 and His247 as ligands for the second ion. Asp97 is a common ligand to both ions. What appears to be a phosphate anion is bound to both zinc atoms, replacing the water molecule/hydroxide ion normally found in this class of enzyme. See details of SGAP in [[Streptomyces griseus Aminopeptidase (SGAP)]]. |
| - | </StructureSection> | |
| | | | |
| | == 3D Structures of Aminopeptidase == | | == 3D Structures of Aminopeptidase == |
| | + | [[Aminopeptidase 3D structures]] |
| | | | |
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}}
| + | </StructureSection> |
| - | {{#tree:id=OrganizedByTopic|openlevels=0|
| + | |
| - | | + | |
| - | *Cysteine aminopeptidase
| + | |
| - | | + | |
| - | **[[3pw3]] – PdCys-AP – ''Parabacteroides distasonis''
| + | |
| - | | + | |
| - | *Glutamic acid aminopeptidase
| + | |
| - | | + | |
| - | **[[2wyr]] – PhGlu-AP+Co – ''Pyrococcus horikoshii''<br />
| + | |
| - | **[[3kl9]] – SpGlu-AP – ''Streptococcus pneumoniae''<br />
| + | |
| - | **[[4kx7]] – hGlu-AP + Zn – human<br />
| + | |
| - | **[[4kx8]], [[4kxb]] – hGlu-AP + Zn + statin derivative<br />
| + | |
| - | **[[4kx9]], [[4kxa]], [[4kxc]], [[4kxd]] – hGlu-AP + Zn + amino acid<br />
| + | |
| - | | + | |
| - | *Alanine aminopeptidase
| + | |
| - | | + | |
| - | **[[3ebg]] – PfAla-AP - ''Plasmodium falciparum''<br />
| + | |
| - | **[[3ebh]] - PfAla-AP+bestatin<br />
| + | |
| - | **[[3ebi]] - PfAla-AP+dipeptide analog<br />
| + | |
| - | **[[4r5t]], [[4r5v]], [[4r5x]] – PfAla-AP + inhibitor <br />
| + | |
| - | **[[4fke]] – pAla-AP + Zn – pig<br />
| + | |
| - | **[[4fkh]] – pAla-AP + Zn + alanine<br />
| + | |
| - | **[[4h5h]], [[4hol]], [[4naq]], [[4nz8]] – pAla-AP + Zn + polyalanine<br />
| + | |
| - | **[[4hom]] – pAla-AP + Zn + substance P<br />
| + | |
| - | **[[4fkk]] – pAla-AP + Zn + bestatin<br />
| + | |
| - | **[[4f5c]] – pAla-AP + PRCV spike protein + Zn<br />
| + | |
| - | **[[4fyq]] - hAla-AP + Zn <br />
| + | |
| - | **[[4fyr]] - hAla-AP + Zn + bestatin<br />
| + | |
| - | **[[4fys]] - hAla-AP + Zn + angiotensin<br />
| + | |
| - | **[[4fyt]] - hAla-AP + Zn + amastatin<br />
| + | |
| - | **[[3ked]] – EcAPN+2,4-diaminobutyric acid – ''Escherichia coli''<br />
| + | |
| - | **[[2hpo]], [[2dq6]], [[3puu]] – EcAPN<br />
| + | |
| - | **[[2hpt]], [[2dqm]] – EcAPN+bestatin<br />
| + | |
| - | **[[2zxg]] – EcAPN+transition state analog<br />
| + | |
| - | **[[3b2p]], [[3b2x]], [[3b34]], [[3b37]], [[3b3b]], [[3qjx]] – EcAPN+amino acid<br />
| + | |
| - | **[[2gtq]] – NmAPN – ''Neisseria meningitides''
| + | |
| - | | + | |
| - | *Proline aminopeptidase
| + | |
| - | | + | |
| - | **[[3ovk]] – Xaa Pro-AP – ''Streptococcus pyogenes''<br />
| + | |
| - | **[[3il0]] - Xaa Pro-AP – ''Streptococcus thermophilus''<br />
| + | |
| - | **[[2v3x]], [[2v3y]], [[2v3z]] - Xaa EcPro-AP (mutant)+tripeptide<br />
| + | |
| - | **[[1w7v]], [[2bh3]] - Xaa EcPro-AP+Zn+Mg+polypeptide<br />
| + | |
| - | **[[2bn7]] - Xaa EcPro-AP+Zn+Mg+Mn+polypeptide<br />
| + | |
| - | **[[2bha]], [[2bhd]] - Xaa EcPro-AP+Mg+polypeptide<br />
| + | |
| - | **[[1a16]] - Xaa EcPro-AP+Mn+polypeptide<br />
| + | |
| - | **[[1wbq]], [[2bhb]] - Xaa EcPro-AP+Zn+Mg<br />
| + | |
| - | **[[2bhc]] - Xaa EcPro-AP+Na+Mg<br />
| + | |
| - | **[[1wl6]] - Xaa EcPro-AP+Mg<br />
| + | |
| - | **[[1wl9]], [[1m35]], [[1jaw]] - Xaa EcPro-AP+Mn<br />
| + | |
| - | **[[1wlr]] - Xaa EcPro-AP<br />
| + | |
| - | **[[2bws]], [[2bwt]], [[2bwu]], [[2bwv]], [[2bww]], [[2bwx]], [[2bwy]] - Xaa EcPro-AP (mutant)<br />
| + | |
| - | **[[1w2m]] - Xaa EcPro-AP+Ca<br />
| + | |
| - | **[[1n51]] – Xaa EcPro-AP+apstatin<br />
| + | |
| - | **[[3ig4]] - Xaa Pro-AP+ Mn – ''Bacillus anthracis''<br />
| + | |
| - | **[[4fkc]] – Xaa Pro-AP + Cd – ''Thermococcus sibiricus''<br />
| + | |
| - | 4pv4]] – Pro-AP II + Mg – ''Yersinia pestis''<br />
| + | |
| - | **[[2zsg]] – X TmPro-AP – ''Thermatoga maritima''<br />
| + | |
| - | **[[3ctz]] – X hPro-AP <br />
| + | |
| - | **[[1x2b]], [[1x2e]], [[1wm1]] – SmPro-AP + inhibitor – ''Serratia marcescens''<br />
| + | |
| - | **[[1qtr]] – SmPro-AP<br />
| + | |
| - | **[[1xqv]] – TaPro-AP (mutant) – ''Thermoplasma acidophilum''<br />
| + | |
| - | **[[1xqw]], [[1xqx]], [[1xqy]], [[1xrl]], [[1xrm]], [[1xrn]], [[1xro]], [[1xrp]], [[1xrq]], [[1xrr]] – TaPro-AP+polypeptide<br />
| + | |
| - | **[[3azo]] – SmPro-AP – ''Streptomyces morookaensis''<br />
| + | |
| - | **[[3azp]] - SmPro-AP (mutant)<br />
| + | |
| - | **[[3azq]] - SmPro-AP (mutant) + PGG<br />
| + | |
| - | **[[1lns]] – Pro-dipeptidyl-AP – ''Lactococcus lactis''<br />
| + | |
| - | **[[2z3w]] – PgPro-tripeptidyl-AP (mutant) – ''Porphyromonas gingivalis''<br />
| + | |
| - | **[[2z3z]] – PgPro-tripeptidyl-AP (mutant) + inhibitor<br />
| + | |
| - | | + | |
| - | *Leucine aminopeptidase
| + | |
| - | | + | |
| - | **[[3jru]] – Leu-AP – ''Xanthomonas oryzae''<br />
| + | |
| - | **[[2hc9]], [[2hb6]] – Leu-AP – ''Caenorhabditis elegans''<br />
| + | |
| - | **[[2ewb]] – bLeu-AP+zofenoprilat – bovine<br />
| + | |
| - | **[[1lam]], [[1lap]] – bLue-AP<br />
| + | |
| - | **[[1bpm]], [[1bpn]] – bLue-AP+Zn+Mg<br />
| + | |
| - | **[[2j9a]] - bLue-AP+Zn + inhibitor<br />
| + | |
| - | **[[1lan]], [[1lcp]] – bLue-AP+leucine derivative<br />
| + | |
| - | **[[1bll]] – bLue-AP+amastatin<br />
| + | |
| - | **[[3rjo]] - hLeu-AP peptide-binding domain<br />
| + | |
| - | **[[3mdj]] - hLeu-AP soluble domain + inhibitor<br />
| + | |
| - | **[[3h8e]], [[3h8f]], [[3h8g]] – Leu-AP – ''Pseudomonas putida''<br />
| + | |
| - | **[[3kqx]], [[3kqz]], [[3kr4]], [[3kr5]] – PfLeu-AP <br />
| + | |
| - | **[[3fh4]], [[1rtq]], [[2dea]] – VpLeu-AP – Vibrio proteolyticus<br />
| + | |
| - | **[[3b35]], [[3b3t]], [[3b3v]], [[2anp]] - VpLeu-AP (mutant)<br />
| + | |
| - | **[[3b3c]], [[3b3s]], [[3b3w]] - VpLeu-AP (mutant)+Leu derivative<br />
| + | |
| - | **[[3b7i]] - VpLeu-AP (mutant)+Leu<br />
| + | |
| - | **[[1ft7]] - VpLeu-AP +Leu derivative<br />
| + | |
| - | **[[3vh9]] - VpLeu-AP + 8-quinolinol<br />
| + | |
| - | **[[2nyq]], [[2iq6]] – VpLeu-AP+polypeptide<br />
| + | |
| - | **[[1lok]] – VpLeu-AP+Tris<br />
| + | |
| - | **[[2prq]] – VpLeu-AP+Co<br />
| + | |
| - | **[[1xry]], [[1txr]] – VpLeu-AP+bestatin<br />
| + | |
| - | **[[1gyt]] – EcLeu-AP<br />
| + | |
| - | **[[3qnf]] – hLeu-AP 1<br />
| + | |
| - | **[[3t8w]], [[4k3n]], [[4r6t]] – PfLeu-AP + Zn + inhibitor <br />
| + | |
| - | **[[4r76]], [[4r7m]] – PfLeu-AP (mutant) + Zn + inhibitor <br />
| + | |
| - | **[[3tc8]] - PdLeu-AP + Zn<br />
| + | |
| - | **[[4fuu]] - Leu-AP + Zn – ''Bacterioides thetaiotaomicron''<br />
| + | |
| - | **[[4ksi]] – Leu-AP + Mg – tomato<br />
| + | |
| - | | + | |
| - | *Leucine-cysteine aminopeptidase
| + | |
| - | | + | |
| - | **[[4p8q]] – hLeu-Cys-AP + Zn<br />
| + | |
| - | **[[4pj6]] – hLeu-Cys-AP + Zn + lysine<br />
| + | |
| - | | + | |
| - | | + | |
| - | *Methionine aminopeptidase
| + | |
| - | | + | |
| - | **[[3mr1]], [[3mx6]] – Met-AP+Mn – ''Rickettsia prowazekii''<br />
| + | |
| - | **[[2dfi]], [[1xgm]], [[1xgn]], [[1xgo]], [[1xgs]] – PfMet-AP+Co – ''Pyrococcus furiosus''<br />
| + | |
| - | **[[1wkm]] - PfMet-AP+Mn<br />
| + | |
| - | **[[4fo7]], [[4juq]] - PaMet-AP+Mn – ''Pseudomonas aeruginosa''<br />
| + | |
| - | **[[4fo8]] - PaMet-AP+Mn + Met <br />
| + | |
| - | **[[4hxx]], [[4iu6]] - hMet-AP+ Co + pyrimidine derivative<br />
| + | |
| - | **[[3iu7]] – MtMet-AP+Mn +A02 – ''Mycobacterium tuberculosis''<br />
| + | |
| - | **[[3iu8]], [[3iu9]] - MtMet-AP+Ni + inhibitor<br />
| + | |
| - | **[[1yj3]], [[3ror]] - MtMet-AP+Co<br />
| + | |
| - | **[[3pka]] - MtMet-AP+Mn<br />
| + | |
| - | **[[3pkb]], [[3pkc]], [[3pkd]], [[3pke]] - MtMet-AP+bengamide inhibitor<br />
| + | |
| - | **[[3tav]] – Met-AP + Mg – ''Mycobacterium abscessus''< br />
| + | |
| - | **[[1y1n]] - MtMet-AP+K<br />
| + | |
| - | **[[4idy]], [[4iec]] - MtMet-AP+K + hydroxyethyl disulfide<br />
| + | |
| - | **[[4if7]] - MtMet-AP+K + homocysteinemethyl disulfide<br />
| + | |
| - | **[[3fm3]] – EncMet-AP – ''Encephalitozoon cuniculi''<br />
| + | |
| - | **[[3fmq]], [[3fmr]] - EncMet-AP+angiogenesis inhibitor<br />
| + | |
| - | **[[3d27]], [[2q92]], [[2q93]], [[2q94]], [[2q95]], [[2q96]], [[2p98]], [[2p99]], [[2p9a]], [[2gu4]], [[2gu5]], [[2gu6]], [[2evc]], [[2evm]], [[2evo]], [[2bbv]], [[1xnz]], [[4a6v]], [[4a6w]], [[2bb7]] – EcMet-AP+Mn+inhibitor<br />
| + | |
| - | **[[2gg0]], [[2gg2]], [[2gg3]], [[2gg5]], [[2gg7]], [[2gg8]], [[2gg9]], [[2ggb]], [[2ggc]], [[4pnc]] - EcMet-AP+Co+inhibitor<br />
| + | |
| - | **[[1c21]], [[1c22]], [[1c23]], [[1c24]] - EcMet-AP +Co + methionine derivative<br />
| + | |
| - | **[[1c27]] - EcMet-AP +Co +norleucine<br />
| + | |
| - | **[[3tb5]] – Met-AP – ''Enterococcus faecalis''<br />
| + | |
| - | **[[2ea2]], [[2ea4]], [[2ga2]], [[2nq6]], [[2nq7]], [[1yw7]], [[1yw8]], [[1yw9]] - hMet-AP+Mn+inhibitor<br />
| + | |
| - | **[[2gtx]], [[2gu7]] – EcMet-AP<br />
| + | |
| - | **[[1yvm]] – EcMet-AP (mutant)+Co+thiabendazole<br />
| + | |
| - | **[[1mat]] – EcMet-AP+Co<br />
| + | |
| - | **[[2mat]], [[4mat]] - EcMet-AP (mutant)+Co<br />
| + | |
| - | **[[3mat]] - EcMet-AP (mutant)+Co+bestatin derivative<br />
| + | |
| - | **[[2b3h]], [[2b3k]] - hMet-AP+Co<br />
| + | |
| - | **[[1boa]] - hMet-AP+Co+ angiogenesis inhibitor<br />
| + | |
| - | **[[2b3l]] – hMet-AP<br />
| + | |
| - | **[[1kq0]], [[1kq9]] – hMet-AP+methionine<br />
| + | |
| - | **[[2gz5]], [[2adu]], [[1qzy]], [[1b59]], [[1b6a]], [[1bn5]], [[2oaz]], [[4ikr]], [[4iks]], [[4ikt]], [[4iku]] – hMet-AP+Co+inhibitor<br />
| + | |
| - | **[[1r58]], [[1r5g]], [[1r5h]], [[4fli]], [[4flj]], [[4flk]], [[4fll]] - hMet-AP+Mn+inhibitor<br />
| + | |
| - | **[[2g6p]] - hMet-AP truncated+Mn+inhibitor<br />
| + | |
| - | **[[1qxw]], [[1qxy]], [[1qxz]] – Met-AP+Co+inhibitor - ''Staphylococcus aureus''<br />
| + | |
| - | **[[1o0x]] – TmMet-AP<br />
| + | |
| - | **[[3s6b]] – PfMet-AP + Fe <br />
| + | |
| - | **[[4fuk]] – TbMet-AP + Zn – ''Trypanosoma brucei''<br />
| + | |
| - | **[[4km3]] – SpAP<br />
| + | |
| - | | + | |
| - | *Arginine aminopeptidase
| + | |
| - | | + | |
| - | **[[4jbs]] – hArg-AP+ Zn + inhibitor<br />
| + | |
| - | | + | |
| - | *Aspartic acid aminopeptidase
| + | |
| - | | + | |
| - | **[[3l6s]], [[4dyo]] – hAsp-AP+aspartic hydroxamate<br />
| + | |
| - | **[[3var]], [[3vat]] – bAsp-AP<br />
| + | |
| - | **[[4eme]] – PfAsp-AP + Zn
| + | |
| - | | + | |
| - | *Asparagine aminopeptidase
| + | |
| - | | + | |
| - | **[[3c17]], [[2zak]] – EcAsn-AP (mutant)<br />
| + | |
| - | **[[2zal]] – EcAsn-AP+Asp<br />
| + | |
| - | **[[2gez]] – Asn-AP – ''Lupinus luteus''
| + | |
| - | | + | |
| - | *Serine aminopeptidase
| + | |
| - | | + | |
| - | **[[1b65]] – OaSer-AP – ''Ochrobactrum anthropi''
| + | |
| - | | + | |
| - | *Cytosolic aminopeptidase
| + | |
| - | | + | |
| - | **[[3pei]] – Cyt-AP – ''Francisella tularensis''<br />
| + | |
| - | **[[3kzw]] – Cyt-AP – ''Staphylococcus aureus''<br />
| + | |
| - | **[[3ij3]] – Cyt-AP – ''Coxiella burnetii''
| + | |
| - | | + | |
| - | *Aminopeptidase 2
| + | |
| - | | + | |
| - | **[[3se6]] – hAPN<br />
| + | |
| - | **[[4e36]] – hAPN + Zn
| + | |
| - | | + | |
| - | *Non-specific aminopeptidase
| + | |
| - | | + | |
| - | **[[2ek8]] – AnAP – Aneurinibacillus<br />
| + | |
| - | **[[2ek9]] – AnAP+bestatin<br />
| + | |
| - | **[[1y0r]], [[1xfo]] – PhAP<br />
| + | |
| - | **[[1y0y]] – PhAP+amastatin<br />
| + | |
| - | **[[1amp]] - VpAP<br />
| + | |
| - | **[[1cp6]], [[1igb]] – VpAP+inhibitor<br />
| + | |
| - | **[[1ei5]] – OaAP<br />
| + | |
| - | **[[3edy]], [[3ee6]] – hTripeptidyl-AP <br />
| + | |
| - | **[[3zn8]] – Dipeptidyl-AP B + signal recognition particle protein + signal recognition particle receptor + RNA – yeast<br />
| + | |
| - | **[[4efd]] – TbAP M17 + Mn<br />
| + | |
| - | **[[4fgm]] – AP N + Zn – ''Idiomarina loihiensis''<br />
| + | |
| - | **[[4icq]] – SpAP Peps + Zn<br />
| + | |
| - | **[[4icr]] – SpAP Peps (mutant) + Zn<br />
| + | |
| - | **[[4ics]] – SpAP Peps (mutant) + Zn + Trp + Gly<br />
| + | |
| - | **[[4pu2]], [[4pvb]] – NmAP N + Leu analog <br />
| + | |
| - | **[[4pw4]] – NmAP N + Phe analog <br />
| + | |
| - | **[[4qhp]], [[4qir]], [[4qme]], [[4qpe]], [[4quo]] – NmAP N + dipeptide analog <br />
| + | |
| - | | + | |
| - | *Cold-activated aminopeptidase
| + | |
| - | | + | |
| - | **[[3cia]] – Col-AP – ''Colwellia psychrerythraea''
| + | |
| - | | + | |
| - | *Deblocking aminopeptidase
| + | |
| - | | + | |
| - | **[[2gre]] – DAP – ''Bacillus cereus''
| + | |
| - | | + | |
| - | *Heat stable aminopeptidase
| + | |
| - | | + | |
| - | **[[2ayi]] – AmpT – ''Thermus thermophilus''
| + | |
| - | | + | |
| - | *Aminopeptidase from ''Staphylococcus aureus''
| + | |
| - | | + | |
| - | **[[1zjc]] - AmpS
| + | |
| - | | + | |
| - | *Metalloaminopeptidase
| + | |
| - | | + | |
| - | **[[3q43]], [[3q44]] – PfPFAP (mutant) + bestatin derivative<br />
| + | |
| - | | + | |
| - | *''Stereomyces griesus'' aminopeptidase
| + | |
| - | | + | |
| - | **[[1xjo]], [[1cp7]] - SGAP<br />
| + | |
| - | **[[1qq9]], [[1f2o]], [[1f2p]], [[1tf8]], [[1tf9]], [[1tkf]], [[1tkh]], [[1tkj]], [[1xbu]] - SGAP + amino acid
| + | |
| - | | + | |
| - | *β-peptidyl aminopeptidase
| + | |
| - | | + | |
| - | **[[3n2w]] – SxBapA – ''Sphingosinicella xenopeptidilytica''<br />
| + | |
| - | **[[3n5i]] – SxBapA (mutant)<br />
| + | |
| - | **[[3n33]] – SxBapA + AEBSF<br />
| + | |
| - | **[[3ndv]], [[3nfb]] – SxBapA + ampicillin
| + | |
| - | | + | |
| - | *M1 family aminopeptidase
| + | |
| - | | + | |
| - | **[[4j3b]] – PfAP (mutant)<br />
| + | |
| - | **[[3t8v]] – PfAP M1<br />
| + | |
| - | **[[4k5l]], [[4k5m]], [[4k5n]], [[4k5p]], [[4k5o]] - PfLeu-AP (mutant) + Zn + inhibitor<br />
| + | |
| | | | |
| - | *Aminopeptidase C | |
| | | | |
| - | **[[4k7c]] – AP – ''Lactobacillus rahmnosis''<br /> | |
| - | }} | |
| | {{Clear}} | | {{Clear}} |
| | | | |