We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ferredoxin
From Proteopedia
(Difference between revisions)
| (12 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='2z8q' size='450' side='right' scene='' caption='Ferredoxin with Fe4S4 cluster complex with cobalt hexamine (PDB code [[2z8q]])'> | <StructureSection load='2z8q' size='450' side='right' scene='' caption='Ferredoxin with Fe4S4 cluster complex with cobalt hexamine (PDB code [[2z8q]])'> | ||
| - | [[Image:2z8q.png|left|150px]]<br /> | ||
| - | + | ==Function== | |
| - | + | [[Ferredoxin]] (Fd) is found in chloroplasts which mediates electron transfer and contains an iron-sulfur cluster. It is involved in the photosynthesis process where its iron atoms accept or discharge electrons when they are being oxidized or reduced. The iron-sulfur cluster can contain 2Fe-2S and is termed plant-like or 3Fe-4S or 4Fe-4S clusters. | |
| + | *'''Adrenodoxin''' (ADR) is a ferredoxin containing a 2Fe-2S group involved in electron transfer from NADPH+ to a cytochrome P-450 in the adrenal gland<ref>PMID:22556163</ref>.. | ||
| + | *'''Putidaredoxin''' (PUT) and '''terpredoxin''' (TER) are involved in the same reaction in bacteria and contain a 2Fe-2S group<ref>PMID:10220356</ref>. | ||
==D14C variant of ''Pyrococcus furiosus'' ferredoxin<ref>DOI 10.1007/s00775-011-0778-7</ref>== | ==D14C variant of ''Pyrococcus furiosus'' ferredoxin<ref>DOI 10.1007/s00775-011-0778-7</ref>== | ||
| Line 27: | Line 28: | ||
The crystal structure of the ''Pyrococcus furiosus'' (Pf) ferredoxin (Fd) D14C variant with the novel [AgFe<sub>3</sub>S<sub>4</sub>] heterometallic cluster was determined to 1.95 Å resolution (PBD entry [[4dhv]]), being the first reported structure of an engineered heterometallic iron-sulfur protein. | The crystal structure of the ''Pyrococcus furiosus'' (Pf) ferredoxin (Fd) D14C variant with the novel [AgFe<sub>3</sub>S<sub>4</sub>] heterometallic cluster was determined to 1.95 Å resolution (PBD entry [[4dhv]]), being the first reported structure of an engineered heterometallic iron-sulfur protein. | ||
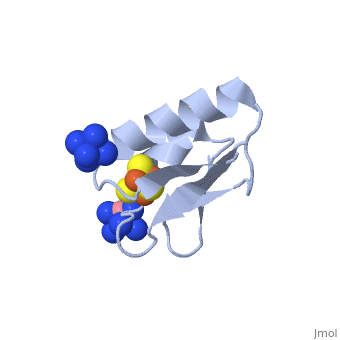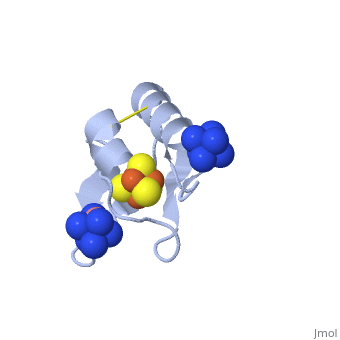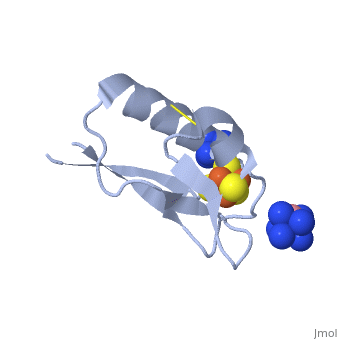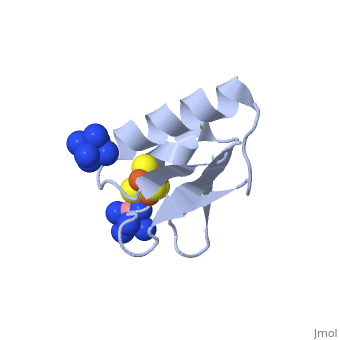
The crystal structure of the <scene name='Journal:JBIC:19/Cv/4'>monomeric form</scene> shows that the <scene name='Journal:JBIC:19/Cv/6'>silver (I) ion is part of the cluster</scene> (clearly seen on the electron density map), as predicted from previous spectroscopic and electrochemical studies. The heterometal is coordinated to the <scene name='Journal:JBIC:19/Cv/5'>three inorganic sulfides of the cluster</scene> and to the <scene name='Journal:JBIC:19/Cv/7'>thiolate group of residue 14</scene> (residues <scene name='Journal:JBIC:19/Cv/8'>Cys11, Cys17 and Cys56</scene> are coordinated with Fe ions of heterometal), <scene name='Journal:JBIC:19/Cv/9'>replacing the aboriginal Fe ion in the all-iron coordinated</scene> [Fe<sub>4</sub>S<sub>4</sub>] D14C variant ([[2z8q]], <span style="color:cyan;background-color:black;font-weight:bold;">heterometallic [AgFe<sub>3</sub>S<sub>4</sub>] protein is in cyan</span> and <span style="color:lime;background-color:black;font-weight:bold;">homometallic [Fe<sub>4</sub>S<sub>4</sub>] is in green</span>) and completing the incomplete cuboidal cluster present in the [Fe<sub>3</sub>S<sub>4</sub>] WT Pf Fd (PDB: [[1sj1]]) and its D14C (PDB: [[3pni]]) variant (for more details see also [http://proteopedia.org/w/Journal:JBIC:10 "Crystal structures of the all cysteinyl coordinated D14C variant of ''Pyrococcus furiosus'' ferredoxin: (4Fe-4S) <-> (3Fe-4S) cluster conversion"]). Structure alignment of backbone atoms from the heterometallic [AgFe<sub>3</sub>S<sub>4</sub>] protein and the homometallic [Fe<sub>4</sub>S<sub>4</sub>] D14C variant ([[2z8q]]) shows <scene name='Journal:JBIC:19/Cv/10'>very minor differences</scene>, i.e. the root mean square deviation (RMSD) is 0.4 – 0.7 Å, observed due to the alternate conformation of the main chain atoms, flexible loops and small changes at the N- and C-termini (<span style="color:cyan;background-color:black;font-weight:bold;">heterometallic [AgFe<sub>3</sub>S<sub>4</sub>] protein is in cyan</span> and <span style="color:lime;background-color:black;font-weight:bold;">homometallic [Fe<sub>4</sub>S<sub>4</sub>] is in green</span>). <scene name='Journal:JBIC:19/Cv/14'>More significant difference</scene> can be seen in the superimposed Fe–S clusters (atom colors corresponding to <span style="color:yellow;background-color:black;font-weight:bold;">yellow: S</span>, <span style="color:orange;background-color:black;font-weight:bold;">orange: Fe</span>, <span style="color:gray;background-color:black;font-weight:bold;">gray: Ag</span>) from these two variants, which is due to the presence of the second row transition metal ion (Ag) coordinated to the four S-ligands, i.e. the presence of Ag results in a distorted geometry of the cluster compared to the all-iron arrangement, <scene name='Journal:JBIC:19/Cv/12'>because to the longer</scene> Ag – S bond lengths compared to Fe – S bonds. However, the S – Ag – S <scene name='Journal:JBIC:19/Cv/13'>bond angles</scene> are still close to the expected 90° for a primitive cubic system. | The crystal structure of the <scene name='Journal:JBIC:19/Cv/4'>monomeric form</scene> shows that the <scene name='Journal:JBIC:19/Cv/6'>silver (I) ion is part of the cluster</scene> (clearly seen on the electron density map), as predicted from previous spectroscopic and electrochemical studies. The heterometal is coordinated to the <scene name='Journal:JBIC:19/Cv/5'>three inorganic sulfides of the cluster</scene> and to the <scene name='Journal:JBIC:19/Cv/7'>thiolate group of residue 14</scene> (residues <scene name='Journal:JBIC:19/Cv/8'>Cys11, Cys17 and Cys56</scene> are coordinated with Fe ions of heterometal), <scene name='Journal:JBIC:19/Cv/9'>replacing the aboriginal Fe ion in the all-iron coordinated</scene> [Fe<sub>4</sub>S<sub>4</sub>] D14C variant ([[2z8q]], <span style="color:cyan;background-color:black;font-weight:bold;">heterometallic [AgFe<sub>3</sub>S<sub>4</sub>] protein is in cyan</span> and <span style="color:lime;background-color:black;font-weight:bold;">homometallic [Fe<sub>4</sub>S<sub>4</sub>] is in green</span>) and completing the incomplete cuboidal cluster present in the [Fe<sub>3</sub>S<sub>4</sub>] WT Pf Fd (PDB: [[1sj1]]) and its D14C (PDB: [[3pni]]) variant (for more details see also [http://proteopedia.org/w/Journal:JBIC:10 "Crystal structures of the all cysteinyl coordinated D14C variant of ''Pyrococcus furiosus'' ferredoxin: (4Fe-4S) <-> (3Fe-4S) cluster conversion"]). Structure alignment of backbone atoms from the heterometallic [AgFe<sub>3</sub>S<sub>4</sub>] protein and the homometallic [Fe<sub>4</sub>S<sub>4</sub>] D14C variant ([[2z8q]]) shows <scene name='Journal:JBIC:19/Cv/10'>very minor differences</scene>, i.e. the root mean square deviation (RMSD) is 0.4 – 0.7 Å, observed due to the alternate conformation of the main chain atoms, flexible loops and small changes at the N- and C-termini (<span style="color:cyan;background-color:black;font-weight:bold;">heterometallic [AgFe<sub>3</sub>S<sub>4</sub>] protein is in cyan</span> and <span style="color:lime;background-color:black;font-weight:bold;">homometallic [Fe<sub>4</sub>S<sub>4</sub>] is in green</span>). <scene name='Journal:JBIC:19/Cv/14'>More significant difference</scene> can be seen in the superimposed Fe–S clusters (atom colors corresponding to <span style="color:yellow;background-color:black;font-weight:bold;">yellow: S</span>, <span style="color:orange;background-color:black;font-weight:bold;">orange: Fe</span>, <span style="color:gray;background-color:black;font-weight:bold;">gray: Ag</span>) from these two variants, which is due to the presence of the second row transition metal ion (Ag) coordinated to the four S-ligands, i.e. the presence of Ag results in a distorted geometry of the cluster compared to the all-iron arrangement, <scene name='Journal:JBIC:19/Cv/12'>because to the longer</scene> Ag – S bond lengths compared to Fe – S bonds. However, the S – Ag – S <scene name='Journal:JBIC:19/Cv/13'>bond angles</scene> are still close to the expected 90° for a primitive cubic system. | ||
| - | </StructureSection> | ||
| - | __NOTOC__ | ||
| - | ===3D structures of ferredoxin=== | ||
| - | + | ==3D structures of ferredoxin== | |
| + | [[Ferredoxin 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==References== | ==References== | ||
Current revision
| |||||||||||
References
- ↑ Ewen KM, Ringle M, Bernhardt R. Adrenodoxin--a versatile ferredoxin. IUBMB Life. 2012 Jun;64(6):506-12. PMID:22556163 doi:10.1002/iub.1029
- ↑ Mo H, Pochapsky SS, Pochapsky TC. A model for the solution structure of oxidized terpredoxin, a Fe2S2 ferredoxin from Pseudomonas. Biochemistry. 1999 Apr 27;38(17):5666-75. PMID:10220356 doi:http://dx.doi.org/10.1021/bi983063r
- ↑ Lovgreen MN, Martic M, Windahl MS, Christensen HE, Harris P. Crystal structures of the all-cysteinyl-coordinated D14C variant of Pyrococcus furiosus ferredoxin: [4Fe-4S] <--> [3Fe-4S] cluster conversion. J Biol Inorg Chem. 2011 Apr 12. PMID:21484348 doi:10.1007/s00775-011-0778-7
- ↑ Iwasaki T, Kappl R, Bracic G, Shimizu N, Ohmori D, Kumasaka T. ISC-like [2Fe-2S] ferredoxin (FdxB) dimer from Pseudomonas putida JCM 20004: structural and electron-nuclear double resonance characterization. J Biol Inorg Chem. 2011 Jun 7. PMID:21647778 doi:10.1007/s00775-011-0793-8
- ↑ Martic M, Jakab-Simon IN, Haahr LT, Hagen WR, Christensen HE. Heterometallic [AgFe(3)S (4)] ferredoxin variants: synthesis, characterization, and the first crystal structure of an engineered heterometallic iron-sulfur protein. J Biol Inorg Chem. 2013 Feb;18(2):261-76. doi: 10.1007/s00775-012-0971-3. Epub, 2013 Jan 8. PMID:23296387 doi:10.1007/s00775-012-0971-3
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky, Eran Hodis, Wayne Decatur, David Canner
![pH dependent equilibrium of D14C [3Fe-4S] P. furiosus ferredoxin between protonated and deprotonated monomers and formation of a disulfide bonded dimer from deprotonated monomers. Fd is short for ferredoxin.](/wiki/images/thumb/0/0b/Schem1.png/300px-Schem1.png)
![Skewed orientations of the gmax component (red) with respect to the molecular frame of the [2Fe–2S] cluster of FdxB.](/wiki/images/2/29/FdxBFig8.jpg)
