Monoclonal Antibody
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
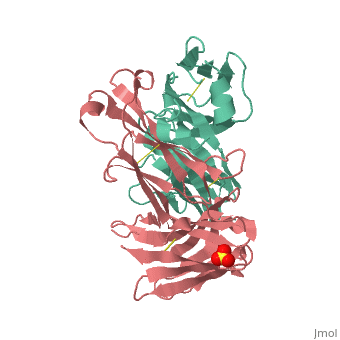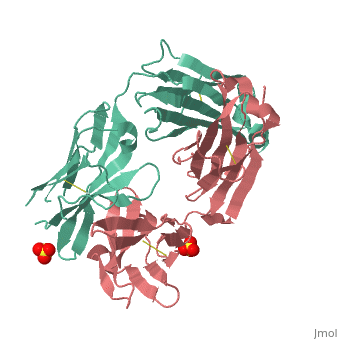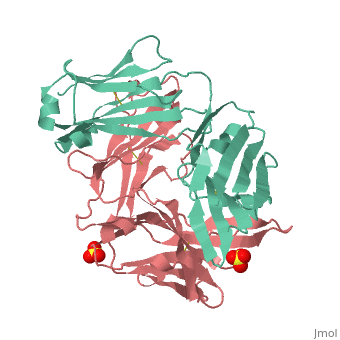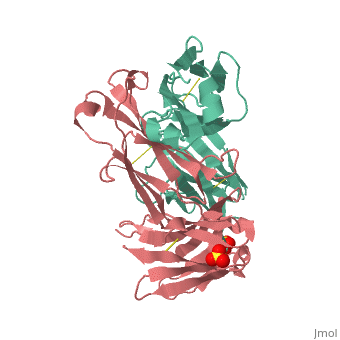
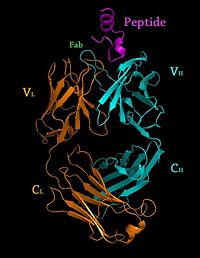
[[Image: Rituxan2.jpg|200px|left|thumb| Crystal Structure of Rituximab Fab in complex with an epitope peptide [[2osl]]]] | [[Image: Rituxan2.jpg|200px|left|thumb| Crystal Structure of Rituximab Fab in complex with an epitope peptide [[2osl]]]] | ||
__TOC__ | __TOC__ | ||
| - | '''Monoclonal antibodies''' are immunoglobulins produced by a single clone of cells and therefore a single pure homogeneous type of antibody. For structural information on immunoglobulins, see: [[Antibody]] The development of antibodies has revolutionized much of the science world both in the lab and in the medical world in treating cancer and autoimmune disorders. Humanized mouse antibody (hmFab) is a modified mFab which resembles more hFab. Catalytic antibody is a | + | '''Monoclonal antibodies''' are immunoglobulins produced by a single clone of cells and therefore a single pure homogeneous type of antibody. For structural information on immunoglobulins, see: [[Antibody]] The development of antibodies has revolutionized much of the science world both in the lab and in the medical world in treating cancer and autoimmune disorders. Humanized mouse antibody (hmFab) is a modified mFab which resembles more hFab. |
| + | *'''Catalytic antibody''' can catalyze certain reactions like an enzyme besides binding to an antigen<ref>PMID:36222989</ref> | ||
| + | *'''Single-chain variable fragment antibody''' or '''scFv''' is a small-size artificial constructs made of the variable regions of the heavy and light chains connected by a peptide linker<ref>PMID:33446839</ref> | ||
<scene name='Monoclonal_Antibody/Opening_monoclonal_antibody/2'>Crystal Structure of the Fab Fragment of Matuzumab</scene>, [[3c08]]. Light (blue) and heavy (yellow) chains. | <scene name='Monoclonal_Antibody/Opening_monoclonal_antibody/2'>Crystal Structure of the Fab Fragment of Matuzumab</scene>, [[3c08]]. Light (blue) and heavy (yellow) chains. | ||
| Line 41: | Line 43: | ||
{{Clear}} | {{Clear}} | ||
===Medical Applications=== | ===Medical Applications=== | ||
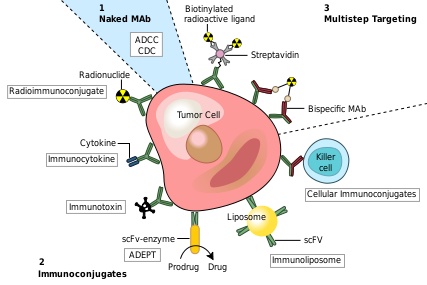
| - | Monoclonal antibodies have been utilized to create four major classes of drugs. The first activate the bodies own immune system. An example of this is [http://en.wikipedia.org/wiki/Rituximab Rituximab] (Marketed as Rituxan by Roche and Genentech). <scene name='Monoclonal_Antibody/Monoclonal_antibody_binding/1'>Rituximab binds</scene> to CD20 surface proteins on B-Cells and illicits natural immune responses to these cancerous cells via [http://en.wikipedia.org/wiki/Antibody-dependent_cellular_cytotoxicity ADCC], activating cellular [http://en.wikipedia.org/wiki/Apoptosis apoptosis], or by attracting compliment proteins which kill the cell via the [http://en.wikipedia.org/wiki/Complement_system compliment pathway.] <ref>PMID:12200395</ref> Another example of this type of monoclonal antibody drug is [http://en.wikipedia.org/wiki/Infliximab Infliximab] (Marketed as [www.remicade.com/ Remicade] by Merck and the best selling Monoclonal therapeutic in 2008 with over $6 billion in sales). See [[Remicade ( | + | Monoclonal antibodies have been utilized to create four major classes of drugs. The first activate the bodies own immune system. An example of this is [http://en.wikipedia.org/wiki/Rituximab Rituximab] (Marketed as Rituxan by Roche and Genentech). <scene name='Monoclonal_Antibody/Monoclonal_antibody_binding/1'>Rituximab binds</scene> to CD20 surface proteins on B-Cells and illicits natural immune responses to these cancerous cells via [http://en.wikipedia.org/wiki/Antibody-dependent_cellular_cytotoxicity ADCC], activating cellular [http://en.wikipedia.org/wiki/Apoptosis apoptosis], or by attracting compliment proteins which kill the cell via the [http://en.wikipedia.org/wiki/Complement_system compliment pathway.] <ref>PMID:12200395</ref> Another example of this type of monoclonal antibody drug is [http://en.wikipedia.org/wiki/Infliximab Infliximab] (Marketed as [www.remicade.com/ Remicade] by Merck and the best selling Monoclonal therapeutic in 2008 with over $6 billion in sales). See [[Remicade (Infliximab)]]. This class of antibodies also includes those antibodies that simply bind a target, disrupting a key mechanism in the diseased cells. |
The second class of monoclonal antibody drugs are those conjugated to a disruptive compound. One example of this is used in Radioimmunotherapy (RIT). By conjugating a radioactive isotope to a murine antibody, targeted immunotherapy is possible. The antibody binds to the targeted cancer cell and the cell is damaged or destroyed by emitted beta particles. Murine antibodies are used because they are cleared from the body rapidly so as to avoid extensive radiation damage from the drug. This is the mechanism used by [http://en.wikipedia.org/wiki/Bexxar Tositumomab] ([http://www.bexxar.com Bexxar]) which uses radioactive Iodine-131. <ref>PMID:17942813</ref> Also within this class of antibodies are those antibodies conjugated to a chemotherapy drug. An example of this type would be [http://en.wikipedia.org/wiki/Mylotarg Gemtuzumab] (marketed as Mylotarg by Wyeth until June 2010, when it was removed from the market due to inefficacy and safety concerns) | The second class of monoclonal antibody drugs are those conjugated to a disruptive compound. One example of this is used in Radioimmunotherapy (RIT). By conjugating a radioactive isotope to a murine antibody, targeted immunotherapy is possible. The antibody binds to the targeted cancer cell and the cell is damaged or destroyed by emitted beta particles. Murine antibodies are used because they are cleared from the body rapidly so as to avoid extensive radiation damage from the drug. This is the mechanism used by [http://en.wikipedia.org/wiki/Bexxar Tositumomab] ([http://www.bexxar.com Bexxar]) which uses radioactive Iodine-131. <ref>PMID:17942813</ref> Also within this class of antibodies are those antibodies conjugated to a chemotherapy drug. An example of this type would be [http://en.wikipedia.org/wiki/Mylotarg Gemtuzumab] (marketed as Mylotarg by Wyeth until June 2010, when it was removed from the market due to inefficacy and safety concerns) | ||
| Line 61: | Line 63: | ||
*For Arzerra see [[Ofatumumab]]<br /> | *For Arzerra see [[Ofatumumab]]<br /> | ||
| - | *For anti-CD20 see [[Rituximab]]<br /> | + | *For anti-CD20 see [[Rituximab]] and [[UMass Chem 423 Student Projects 2011-2]]<br /> |
*For anti-pollen allergen antibody see [[Phl p 2]]<br /> | *For anti-pollen allergen antibody see [[Phl p 2]]<br /> | ||
*For Herceptin anti-EGFR-2 see [[Trastuzumab]]<br /> | *For Herceptin anti-EGFR-2 see [[Trastuzumab]]<br /> | ||
| Line 68: | Line 70: | ||
*[[Remicade (Infliximab)]]<br /> | *[[Remicade (Infliximab)]]<br /> | ||
* [[Tositumomab]] pharmacokinetics. | * [[Tositumomab]] pharmacokinetics. | ||
| - | </StructureSection> | ||
==3D structure of monoclonal antibody== | ==3D structure of monoclonal antibody== | ||
| - | [[ | + | [[3D structures of monoclonal antibody]] |
| + | |||
| + | </StructureSection> | ||
| + | |||
==References== | ==References== | ||
Current revision
| |||||||||||
References
- ↑ Zhao D, Chen J, Hu X, Zhang S. Catalytic Antibodies: Design, Expression, and Their Applications in Medicine. Appl Biochem Biotechnol. 2023 Feb;195(2):1514-1540. PMID:36222989 doi:10.1007/s12010-022-04183-1
- ↑ de Aguiar RB, da Silva TA, Costa BA, Machado MFM, Yamada RY, Braggion C, Perez KR, Mori MAS, Oliveira V, de Moraes JZ. Generation and functional characterization of a single-chain variable fragment (scFv) of the anti-FGF2 3F12E7 monoclonal antibody. Sci Rep. 2021 Jan 14;11(1):1432. PMID:33446839 doi:10.1038/s41598-020-80746-8
- ↑ 3.0 3.1 Roit, I. M. Roit's Essential Immunology. Oxford: Blackwell Science Ltd., 1997.
- ↑ Ghosh AK, Spriggs AI, Taylor-Papadimitriou J, Mason DY. Immunocytochemical staining of cells in pleural and peritoneal effusions with a panel of monoclonal antibodies. J Clin Pathol. 1983 Oct;36(10):1154-64. PMID:6194183
- ↑ Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000 Jun;124(6):921-3. PMID:10835540
- ↑ Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995 Nov;1(11):1311-8. PMID:9815926
- ↑ Brady RL, Edwards DJ, Hubbard RE, Jiang JS, Lange G, Roberts SM, Todd RJ, Adair JR, Emtage JS, King DJ, et al.. Crystal structure of a chimeric Fab' fragment of an antibody binding tumour cells. J Mol Biol. 1992 Sep 5;227(1):253-64. PMID:1522589
- ↑ Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323-7. PMID:3127726 doi:http://dx.doi.org/10.1038/332323a0
- ↑ Barbas CF 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978-82. PMID:1896445
- ↑ Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985 Jun 14;228(4705):1315-7. PMID:4001944
- ↑ Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004 Nov;22(11):1393-8. PMID:15529164 doi:10.1038/nbt1026
- ↑ Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5022-7. Epub 2003 Apr 17. PMID:12702754 doi:10.1073/pnas.0931263100
- ↑ van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002 Sep 15;100(6):2257-9. PMID:12200395
- ↑ Jacene HA, Filice R, Kasecamp W, Wahl RL. Comparison of 90Y-ibritumomab tiuxetan and 131I-tositumomab in clinical practice. J Nucl Med. 2007 Nov;48(11):1767-76. Epub 2007 Oct 17. PMID:17942813 doi:10.2967/jnumed.107.043489
- ↑ Bagshawe KD. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Rev Anticancer Ther. 2006 Oct;6(10):1421-31. PMID:17069527 doi:10.1586/14737140.6.10.1421
- ↑ Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res. 2001 Nov;7(11):3314-24. PMID:11705842
- ↑ http://www.nanotech-now.com/news.cgi?story_id=38872
- ↑ Lippow SM, Wittrup KD, Tidor B. Computational design of antibody-affinity improvement beyond in vivo maturation. Nat Biotechnol. 2007 Oct;25(10):1171-6. Epub 2007 Sep 23. PMID:17891135 doi:10.1038/nbt1336
- ↑ Gribenko AV, Patel MM, Liu J, McCallum SA, Wang C, Makhatadze GI. Rational stabilization of enzymes by computational redesign of surface charge-charge interactions. Proc Natl Acad Sci U S A. 2009 Feb 5. PMID:19196981
- ↑ Ducancel F, Muller BH. Molecular engineering of antibodies for therapeutic and diagnostic purposes. MAbs. 2012 Jul-Aug;4(4):445-57. doi: 10.4161/mabs.20776. Epub 2012 Jul 1. PMID:22684311 doi:http://dx.doi.org/10.4161/mabs.20776
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky