Plasminogen activator
From Proteopedia
(Difference between revisions)
| (17 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
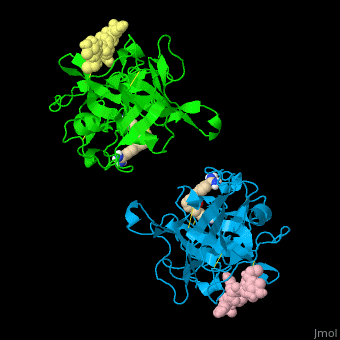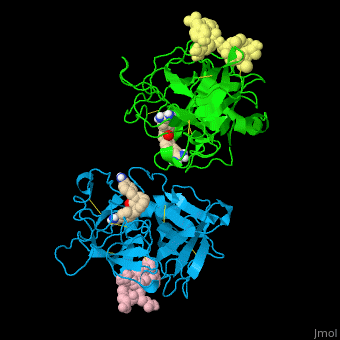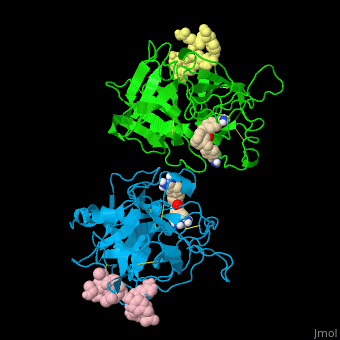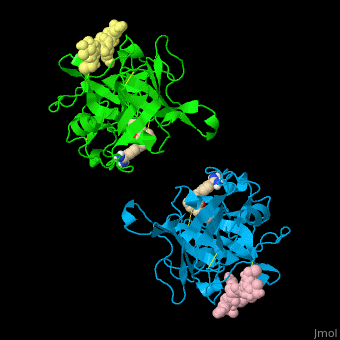
| - | + | <StructureSection load='' size='350' side='right' caption='Human tissue plasminogen activator light chain catalytic domain (sky blue and green), heavy chain catalytic domain fragment (yellow and pink) complex with benzamidine, [[1a5h]]' scene='47/476004/Cv/1' pspeed='8' > | |
| + | == Function == | ||
| + | '''Plasminogen activator''' (PLA) is a serine protease which converts plasminogen to plasmin. TPA structure contains heavy and light chains (HC, LC). PLA is inhibited by PLA inhibitor 1 and 2. | ||
| - | ''' | + | *'''Tissue PLA''' (TPA) which is involved in breakdown of blood clots. |
| + | *'''Urokinase-type PLA''' see [[Urokinase]].<br /> | ||
| + | *'''Coagulase/fibrinolysin''' is a plasminogen activator from ''Yersinia pestis'' which possesses coagulase activity is responsible for degradation of plasmid-coded outer membrane proteins<ref>PMID:2843471</ref>. | ||
| - | + | == Relevance == | |
| - | + | Treatment with tissue PLA for acute ischemic stroke is beneficial<ref>PMID:7477192</ref>. | |
| - | ===3D structures of plasminogen activator | + | == Structural highlights == |
| + | *<scene name='47/476004/Cv/4'>Interactions between tissue plasminogen activator light chain catalytic domain and heavy chain catalytic domain fragment</scene>. Water molecules are shown as red spheres. | ||
| + | *<scene name='47/476004/Cv/5'>Benzamidine binding site</scene>. | ||
| + | </StructureSection> | ||
| + | ==3D structures of plasminogen activator== | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | [[1a5i]] – PLA residues 213-477 + EGR-CMK – Vampire bat<br /> | + | *Plasminogen activator |
| - | [[1bqy]] – PLA – Pit viper | + | |
| + | **[[1a5i]] – PLA residues 213-477 + EGR-CMK – Vampire bat<br /> | ||
| + | **[[1bqy]] – PLA – Pit viper | ||
| - | + | *Tissue plasminogen activator; Domains – fibrin-binding 36-85; kringle 1 69-153; kringle 2 209-298; catalytic 311-561 | |
| + | |||
| + | **[[1a5h]] – hTPA HC+LC + benzamidine – human<br /> | ||
| + | **[[1rtf]] - hTPA HC+LC<br /> | ||
| + | **[[1tpg]] – hTPA F1-G - NMR<br /> | ||
| + | **[[1pk2]] – hTPA kringle 2 domain + 6-aminohexanoic acid – NMR<br /> | ||
| + | **[[1tpk]], [[1pml]] - hTPA kringle 2 domain<br /> | ||
| + | **[[1kdu]] - hTPA kringle 1 domain – NMR<br /> | ||
| + | **[[1tpm]], [[1tpn]] - hTPA fibrin-binding domain – NMR<br /> | ||
| + | **[[1bda]] – hTPA catalytic domain (mutant) + EGR-CMK<br /> | ||
| + | **[[5brr]], [[5zlz]] - hTPA catalytic domain (mutant) + plasminogen activator inhibitor 1<br /> | ||
| - | + | *Coagulase/fibrinolysin | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | **[[2x55]], [[2x56]] - YpTPA – ''Yersinia pestis''<br /> | |
| + | **[[2x4m]] - YpTPA (mutant)<br /> | ||
| + | **[[4dcb]] - YpTPA (mutant) + plasminogen peptide<br /> | ||
| - | [[Urokinase]] | ||
| + | *Urokinase-type plasminogen activator see [[Urokinase]] | ||
| + | }} | ||
| + | == References == | ||
| + | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of plasminogen activator
Updated on 21-July-2024
References
- ↑ Sodeinde OA, Sample AK, Brubaker RR, Goguen JD. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988 Oct;56(10):2749-52. PMID:2843471
- ↑ . Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995 Dec 14;333(24):1581-7. PMID:7477192 doi:http://dx.doi.org/10.1056/NEJM199512143332401