RNA uridylyltransferase
From Proteopedia
(Difference between revisions)
| (18 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
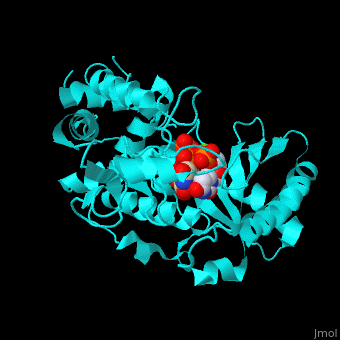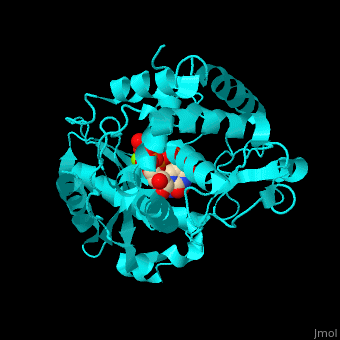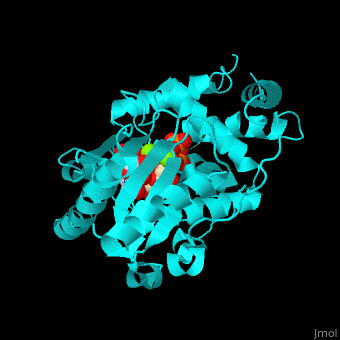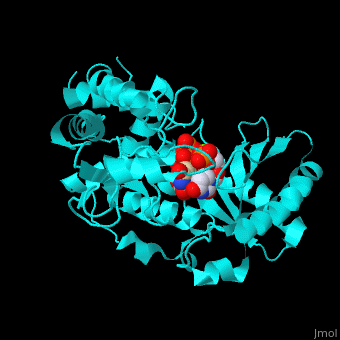
| - | + | <StructureSection load='' size='350' side='right' caption='RNA uridylyltransferase-4 complex with UTP, UMP and Mg+2 ions (green) (PDB entry [[2q0f]])' scene='53/536705/Cv/1'> | |
| - | + | == Function == | |
| + | '''RNA uridylyltransferase''' or '''terminal uridylyltransferase''' or '''TUTase''' (TUT) catalyzes the addition of uridine to RNA. TUT acts as a suppressor of microRNA synthesis by mediating its terminal uridylation<ref>PMID:17189640</ref>. For additional details on TUT-4 see [[Terminal Uridylyl Transferase]]. | ||
| + | * ''Drosophila melanogaster'' TUTase is called '''TUTase Tailor'''. It is forming the uridylation-mediated processing complex which degrades a variety ofncRNAs in the cytoplasm <ref>PMID:26145174</ref>,.<ref>PMID:27729457</ref> | ||
| + | == Structural highlights == | ||
| + | <scene name='53/536705/Cv/5'>TUT-4 active site contains UTP, UMP and Mg+2 ions</scene><ref>PMID:17785418</ref>. Water molecules are shown as red spheres. <scene name='53/536705/Cv/6'>Mg coordination site</scene>. | ||
| + | </StructureSection> | ||
| + | == 3D Structures of RNA uridylyltransferase == | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | *RNA uridylyltransferase-1 | ||
| + | **[[2e5g]] – hTUT-1 RNA recognition motif – human – NMR<br /> | ||
| + | **[[3pq1]] – hTUT-1 (mutant)<br /> | ||
| + | **[[6l3f]] – AtTUT-1 (mutant) – ''Arabidopsis thaliana''<br /> | ||
| + | **[[6l8k]] – AtTUT-1 + UTP<br /> | ||
| + | **[[2b51]], [[5hcd]] – TbTUT-1 – ''Trypanosoma brucei''<br /> | ||
| + | **[[5ido]] – TbTUT-1 + UTP <br /> | ||
| + | **[[5i49]] – TbTUT-1 + UMPNPP <br /> | ||
| + | *RNA uridylyltransferase-4 | ||
| + | **[[6iw6]] – hTUT-4 253-723<br /> | ||
| + | **[[2q0c]], [[2q0d]], [[2q0e]], [[2q0f]], [[2q0g]], [[2ikf]], [[2nom]], [[5kal]] – TbTUT-4 + nucleotide <br /> | ||
| + | *RNA uridylyltransferase-7 | ||
| + | **[[5w0b]] – hTUT-7 catalytic module<br /> | ||
| + | **[[5w0m]] – hTUT-7 + U5 ssRNA <br /> | ||
| + | **[[5w0o]] – hTUT-7 + dsRNA + UTPbr /> | ||
| + | **[[5w0n]] – hTUT-7 + U2 ssRNA + UMPNPP<br /> | ||
| + | *TUTase Tailor | ||
| + | **[[5z4c]] – DmTUTT – ''Drosophila melanogaster'' <br /> | ||
| + | **[[5z4a]], [[5z4d]], [[5z4j]], [[6i0t]], [[6i0u]], [[6i0v]] – DmTUTT + RNA <br /> | ||
| + | **[[5z4m]] – DmTUTT (mutant) + UTP <br /> | ||
| - | + | }} | |
| - | + | == References == | |
| - | + | <references/> | |
| - | + | [[Category:Topic Page]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | |
| - | + | ||
| - | + | ||
| - | [[ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
| |||||||||||
3D Structures of RNA uridylyltransferase
Updated on 11-August-2024
References
- ↑ Stagno J, Aphasizheva I, Rosengarth A, Luecke H, Aphasizhev R. UTP-bound and Apo structures of a minimal RNA uridylyltransferase. J Mol Biol. 2007 Feb 23;366(3):882-99. Epub 2006 Dec 2. PMID:17189640 doi:10.1016/j.jmb.2006.11.065
- ↑ Bortolamiol-Becet D, Hu F, Jee D, Wen J, Okamura K, Lin CJ, Ameres SL, Lai EC. Selective Suppression of the Splicing-Mediated MicroRNA Pathway by the Terminal Uridyltransferase Tailor. Mol Cell. 2015 Jul 16;59(2):217-28. doi: 10.1016/j.molcel.2015.05.034. Epub 2015 , Jul 2. PMID:26145174 doi:http://dx.doi.org/10.1016/j.molcel.2015.05.034
- ↑ Reimão-Pinto MM, Manzenreither RA, Burkard TR, Sledz P, Jinek M, Mechtler K, Ameres SL. Molecular basis for cytoplasmic RNA surveillance by uridylation-triggered decay in Drosophila. EMBO J. 2016 Nov 15;35(22):2417-2434. PMID:27729457 doi:10.15252/embj.201695164
- ↑ Stagno J, Aphasizheva I, Aphasizhev R, Luecke H. Dual role of the RNA substrate in selectivity and catalysis by terminal uridylyl transferases. Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14634-9. Epub 2007 Sep 4. PMID:17785418