Transferrin receptor
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
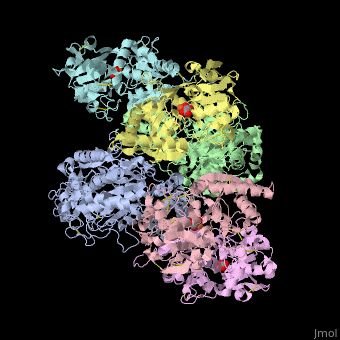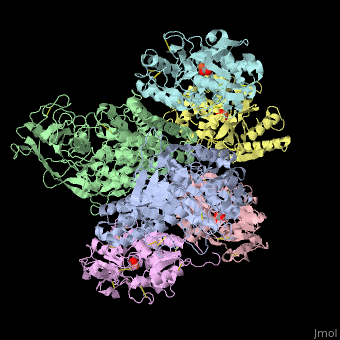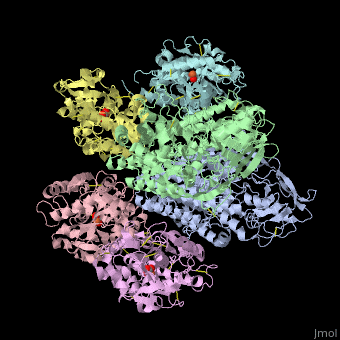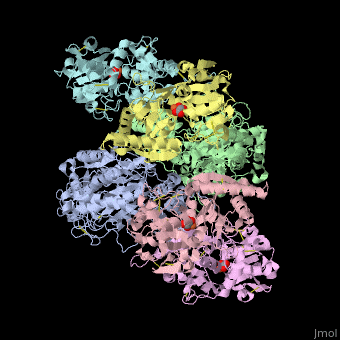
<StructureSection load='' size='340' side='right' caption='Human transferrin receptor ectodomain dimer (grey and green) complex with transferrin N-lobe (pink and yellow) and C-lobe (cyan and magenta) and carbonate, [[1suv]]' scene='48/485628/Cv/1' > | <StructureSection load='' size='340' side='right' caption='Human transferrin receptor ectodomain dimer (grey and green) complex with transferrin N-lobe (pink and yellow) and C-lobe (cyan and magenta) and carbonate, [[1suv]]' scene='48/485628/Cv/1' > | ||
| + | __TOC__ | ||
== Function == | == Function == | ||
'''Transferrin receptor''' (TfR) imports iron into the cell by receptor-mediated endocytosis of [[Transferrin|transferrin]]-iron complex. TfR is regulated by intracellular iron concentration. The amount of TfR expressed on the cell is proportional to the cell’s need for iron. The TfR has selective affinity for diferric-transferrin. The TfR-transferrin complex is internalized by the cell and iron is released to the cytosol. The TfR-apotransferrin complex returns to the extracellular surface where the apotransferrin dissociates and is replaced by diferric-transferrin<ref>PMID:10192390</ref>. | '''Transferrin receptor''' (TfR) imports iron into the cell by receptor-mediated endocytosis of [[Transferrin|transferrin]]-iron complex. TfR is regulated by intracellular iron concentration. The amount of TfR expressed on the cell is proportional to the cell’s need for iron. The TfR has selective affinity for diferric-transferrin. The TfR-transferrin complex is internalized by the cell and iron is released to the cytosol. The TfR-apotransferrin complex returns to the extracellular surface where the apotransferrin dissociates and is replaced by diferric-transferrin<ref>PMID:10192390</ref>. | ||
| Line 7: | Line 8: | ||
== Structural highlights == | == Structural highlights == | ||
*<scene name='48/485628/Cv/3'>Human transferrin receptor ectodomain dimer complex with transferrin N-lobe and C-lobe</scene> ([[1suv]]). | *<scene name='48/485628/Cv/3'>Human transferrin receptor ectodomain dimer complex with transferrin N-lobe and C-lobe</scene> ([[1suv]]). | ||
| - | + | ||
==3D structures of transferrin receptor== | ==3D structures of transferrin receptor== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | Domains – apical 197-378; ectodomain 121-760 | ||
[[1cx8]] – hTfR ectodomain – human<br /> | [[1cx8]] – hTfR ectodomain – human<br /> | ||
| + | [[6y76]], [[8p0z]] - hTfR apical domain <br /> | ||
[[1de4]] - hTfR ectodomain + hemochromatosis protein + β-2-microglobulin<br /> | [[1de4]] - hTfR ectodomain + hemochromatosis protein + β-2-microglobulin<br /> | ||
| - | [[1suv]] - hTfR ectodomain + transferrin<br /> | + | [[1suv]], [[6soy]], [[6soz]] - hTfR ectodomain + transferrin<br /> |
| + | [[6okd]] - hTfR ectodomain + TfR-binding cysteine-dense peptide<br /> | ||
| + | [[6d03]], [[6d04]], [[6d05]] - hTfR ectodomain + transferrin – Cryo EM<br /> | ||
| + | [[6gsr]], [[6h5i]] - hTfR ectodomain + ferritin heavy chain – Cryo EM<br /> | ||
| + | [[6s9j]] - hTfR ectodomain + PRE-GP-C<br /> | ||
[[3s9l]], [[3s9m]], [[3s9n]] - hTfR ectodomain + transferrin N-lobe<br /> | [[3s9l]], [[3s9m]], [[3s9n]] - hTfR ectodomain + transferrin N-lobe<br /> | ||
[[2nsu]] - hTfR ectodomain in dog retrovirus – Cryo EM<br /> | [[2nsu]] - hTfR ectodomain in dog retrovirus – Cryo EM<br /> | ||
[[3kas]] - hTfR ectodomain + Machupo virus GP1<br /> | [[3kas]] - hTfR ectodomain + Machupo virus GP1<br /> | ||
| + | [[6wrv]], [[6wrw]], [[6wrx]] - hTfR ectodomain + synthetic protein<br /> | ||
| + | [[7zqs]] - hTfR + DNA – Cryo EM<br /> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | + | </StructureSection> | |
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||