Electrostatic potential maps
From Proteopedia
(Difference between revisions)
(→Gallery) |
(→Gallery) |
||
| Line 8: | Line 8: | ||
|style="width: 250;" width="250"| [[Image:1pgb-EPM-PyMOL.png|250 px]] | |style="width: 250;" width="250"| [[Image:1pgb-EPM-PyMOL.png|250 px]] | ||
|style="width: 250;" width="250"| [[Image:1pgb-EPM-iCn3D.png|250 px]] | |style="width: 250;" width="250"| [[Image:1pgb-EPM-iCn3D.png|250 px]] | ||
| - | |style="width: 250;" width="250"| [[Image:1pgb- | + | |style="width: 250;" width="250"| [[Image:1pgb-charge-fgij.png|250 px]] |
|- | |- | ||
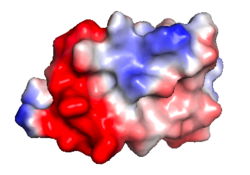
| Electrostatic potential map rendered by [[PyMOL]]. | | Electrostatic potential map rendered by [[PyMOL]]. | ||
Revision as of 18:10, 25 August 2024
It is revealing to visualize the distribution of electrostatic charges, electrostatic potential, on molecular surfaces. Most protein-protein and protein-ligand interactions are largely electrostatic in nature, via hydrogen bonds and ionic interactions. Their strengths are modulated by the nature of the solvent: pure water or high ionic strength aqueous solution.
Gallery
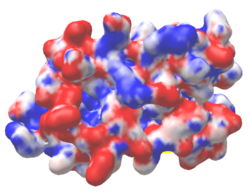
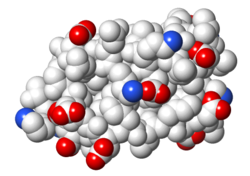
| Protein 1pgb is in the same orientation in all images. | ||
|---|---|---|
| 
| 
|
| Electrostatic potential map rendered by PyMOL. | Electrostatic potential map rendered by iCn3D. | Van der Waals model colored by charge wtih FirstGlance in Jmol. |
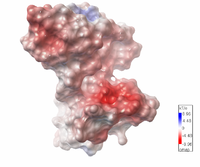 | Electrostatic potential map of 1tsj made with the Embedded Python Molecular Viewer from the Center for Computational Structural Biology of the Scripps Research Institute. |
Click on the image to enlarge. |}
See Also
- Electrostatic interactions in Proteopedia.
- Jmol/Electrostatic potential methods.
- Isopotential Map in Wikipedia
- Delphi Web Server
