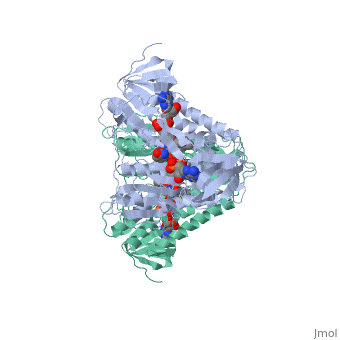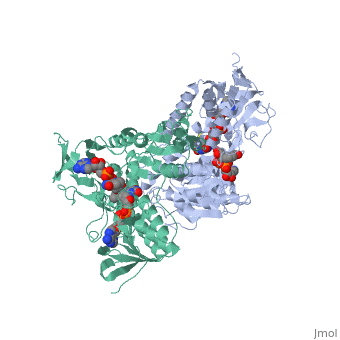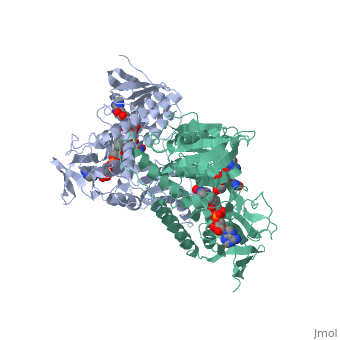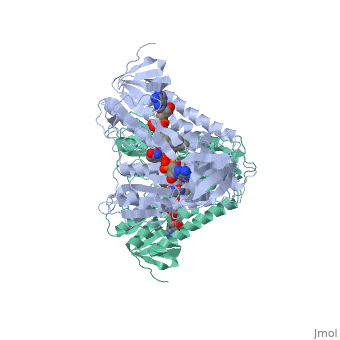
Dihydrolipoamide dehydrogenase
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
==General== | ==General== | ||
| - | '''Dihyrolipoamide dehydrogenase''' (E3) is a part of the multienzyme complex of pyruvate dehydrogenase. This multienzyme complex catalyzes the formation of Acetyl-CoA from pyruvate via oxidative decarboxylation. E3 is a flavoprotein and contains FAD. | + | '''Dihyrolipoamide dehydrogenase''' (E3) is a part of the multienzyme complex of pyruvate dehydrogenase. This multienzyme complex catalyzes the formation of Acetyl-CoA from pyruvate via oxidative decarboxylation. E3 is a flavoprotein and contains FAD. See also [[2-Oxoglutarate Dehydrogenase]] |
==Structure== | ==Structure== | ||
| Line 9: | Line 9: | ||
==Mechanism== | ==Mechanism== | ||
The redox reaction occurs through the influence of <scene name='Nicholas_Rockefeller_Sandbox/Cys43cys48/3'>Cys 43 and Cys 48</scene>, between which there is a disulfide bond within a distorted alpha helix. This redox active disulfide bond becomes reduced in order to reoxidize the E2 enzyme of the multienzyme complex.‘<ref>Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. pp.570-575</ref>’ E2 donates protons and electrons to E3 in order to complete its catalytic cycle. The E3 enzyme’s flavin ring (FAD) funnels electrons from the disulfide bond to itself, <scene name='Nicholas_Rockefeller_Sandbox/Fadnad/1'>then binds to NAD+ and donates electrons</scene>, reoxidizing the E3, and leaving it ready for the beginning of its catalytic cycle again. | The redox reaction occurs through the influence of <scene name='Nicholas_Rockefeller_Sandbox/Cys43cys48/3'>Cys 43 and Cys 48</scene>, between which there is a disulfide bond within a distorted alpha helix. This redox active disulfide bond becomes reduced in order to reoxidize the E2 enzyme of the multienzyme complex.‘<ref>Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. pp.570-575</ref>’ E2 donates protons and electrons to E3 in order to complete its catalytic cycle. The E3 enzyme’s flavin ring (FAD) funnels electrons from the disulfide bond to itself, <scene name='Nicholas_Rockefeller_Sandbox/Fadnad/1'>then binds to NAD+ and donates electrons</scene>, reoxidizing the E3, and leaving it ready for the beginning of its catalytic cycle again. | ||
| + | |||
| + | ==Disease== | ||
| + | DLD deficiency is a rare metabolic disorder causing neurological or liver impairment<ref>PMID:23478190</ref>. | ||
==Regulation== | ==Regulation== | ||
The regulation of Dihidrolipoamide dehydrogenase (E3) kinetically comes through regulation of the entire Pyruvate Dehydrogenase complex. As would be expected, one of the main regulators is the presence of its product, acetyl-CoA as well as NADH. This is through the E1 reaction of the complex, but necessarily effects the E3 reaction. However, the E1 portion of the complex is also regulated by phosphatase and kinase in phosphorylation and dephosphorylation reactions.‘<ref>Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. p.585</ref>’ | The regulation of Dihidrolipoamide dehydrogenase (E3) kinetically comes through regulation of the entire Pyruvate Dehydrogenase complex. As would be expected, one of the main regulators is the presence of its product, acetyl-CoA as well as NADH. This is through the E1 reaction of the complex, but necessarily effects the E3 reaction. However, the E1 portion of the complex is also regulated by phosphatase and kinase in phosphorylation and dephosphorylation reactions.‘<ref>Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. p.585</ref>’ | ||
| - | </StructureSection> | ||
==3D structures of dihydrolipoamide dehydrogenase== | ==3D structures of dihydrolipoamide dehydrogenase== | ||
| Line 21: | Line 23: | ||
*DLD | *DLD | ||
| - | **[[3ic9]] – DLD – ''Colwellia psychrerythraea''<br /> | ||
| - | **[[2qae]] – DLD – ''Trypanosoma cruzi''<br /> | ||
**[[2ihw]] – bDLD E2 - bovine<br /> | **[[2ihw]] – bDLD E2 - bovine<br /> | ||
| - | **[[2eq6]] – TtDLD – ''Thermus thermophilus''<br /> | ||
| - | **[[2yqu]] – TtDLD E3<br /> | ||
| - | **[[2eq7]], [[2eq8]], [[2eq9]] – TtDLD E2+E3<br /> | ||
**[[1ivi]] – DLD – pig<br /> | **[[1ivi]] – DLD – pig<br /> | ||
| - | **[[1jeh]] – yDLD E3 – yeast<br /> | ||
**[[1dxl]] – DLD – pea<br /> | **[[1dxl]] – DLD – pea<br /> | ||
| + | **[[1jeh]] – yDLD E3 – yeast<br /> | ||
**[[1lpf]] – DLD – ''Pseudomonas fluorescens''<br /> | **[[1lpf]] – DLD – ''Pseudomonas fluorescens''<br /> | ||
**[[3lad]] – DLD – ''Azotobacter vinelandii''<br /> | **[[3lad]] – DLD – ''Azotobacter vinelandii''<br /> | ||
| Line 38: | Line 35: | ||
**[[1w4i]] – PaDLD peripheral-subunit binding domain – ''Pyrobaculum aerophilum'' - NMR<br /> | **[[1w4i]] – PaDLD peripheral-subunit binding domain – ''Pyrobaculum aerophilum'' - NMR<br /> | ||
**[[1w4j]], [[1w4k]] – PaDLD peripheral-subunit binding domain (mutant) - NMR<br /> | **[[1w4j]], [[1w4k]] – PaDLD peripheral-subunit binding domain (mutant) - NMR<br /> | ||
| + | **[[3ic9]] – DLD – ''Colwellia psychrerythraea''<br /> | ||
| + | **[[2qae]] – DLD – ''Trypanosoma cruzi''<br /> | ||
| + | **[[2eq6]] – TtDLD – ''Thermus thermophilus''<br /> | ||
| + | **[[2yqu]] – TtDLD E3<br /> | ||
| + | **[[2eq7]], [[2eq8]], [[2eq9]] – TtDLD E2+E3<br /> | ||
*DLD binary complexes | *DLD binary complexes | ||
| - | **[[2ii3]], [[2ii4]], [[2ii5]] – bDLD + CoA<br /> | ||
**[[3rnm]] – hDLD subunit-binding domain + dihydrolipoyl dehydrogenase – human<br /> | **[[3rnm]] – hDLD subunit-binding domain + dihydrolipoyl dehydrogenase – human<br /> | ||
**[[2f5z]], [[1zy8]] – hDLD E3 + pyruvate dehydrogenase E3-binding domain<br /> | **[[2f5z]], [[1zy8]] – hDLD E3 + pyruvate dehydrogenase E3-binding domain<br /> | ||
**[[1zmc]], [[1zmd]] – hDLD + NAD<br /> | **[[1zmc]], [[1zmd]] – hDLD + NAD<br /> | ||
| - | **[[5j5z]] – hDLD (mutant) + FAD <br /> | + | **[[6i4q]] – hDLD + FAD <br /> |
| + | **[[5j5z]], [[6hg8]], [[6i4p]], [[6i4r]], [[6i4s]], [[6i4t]], [[6i4u]], [[6i4z]], [[7psc]], [[7zyt]] – hDLD (mutant) + FAD <br /> | ||
| + | **[[2ii3]], [[2ii4]], [[2ii5]] – bDLD + CoA<br /> | ||
**[[1v59]] – yDLD + NAD<br /> | **[[1v59]] – yDLD + NAD<br /> | ||
| + | **[[1lvl]] – PpDLD + NAD – ''Pseudomonas putida''<br /> | ||
| + | **[[6awa]] - PpDLD + FAD + AMP<br /> | ||
**[[3ii4]] – MtDLD + inhibitor <br /> | **[[3ii4]] – MtDLD + inhibitor <br /> | ||
| - | **[[4m52]] – MtDLD + sulfonamide inhibitor<br /> | + | **[[4m52]], [[8u0q]] – MtDLD + FAD + sulfonamide inhibitor<br /> |
| + | **[[8ct4]] – MtDLD + FAD + sulfonamide inhibitor – Cryo EM<br /> | ||
| + | **[[7kmy]] – MtDLD + FAD + pyridine inhibitor<br /> | ||
**[[1ebd]] – DLD + dihydrolipoamide acetyltransferase binding domain – ''Geobacillus stearothermophilus''<br /> | **[[1ebd]] – DLD + dihydrolipoamide acetyltransferase binding domain – ''Geobacillus stearothermophilus''<br /> | ||
**[[4jdr]] – DLD + FAD – ''Escherichia coli''<br /> | **[[4jdr]] – DLD + FAD – ''Escherichia coli''<br /> | ||
| Line 54: | Line 61: | ||
**[[5u25]] – DLD + FAD – ''Neisseria gonorrhoeae''<br /> | **[[5u25]] – DLD + FAD – ''Neisseria gonorrhoeae''<br /> | ||
**[[6aon]] – DLD + FAD – ''Bordetella pertussis''<br /> | **[[6aon]] – DLD + FAD – ''Bordetella pertussis''<br /> | ||
| + | **[[6cmz]] – DLD + FAD + NAD – ''Burkholderia cenocepacia''<br /> | ||
| + | **[[6uzi]] – DLD + FAD – ''Elizabethkingia anophelis<''br /> | ||
| + | **[[6bz0]] – DLD + FAD – ''Acinetobacter baumannii''<br /> | ||
| + | **[[8ajj]] – DLD + FAD – ''Staphylococcus aureus<''br /> | ||
}} | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | + | </StructureSection> | |
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Nicholas Rockefeller, Alexander Berchansky, David Canner, Shane Michael Evans, Jaime Prilusky