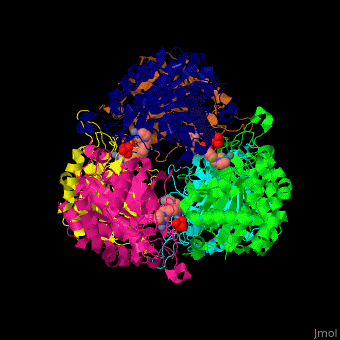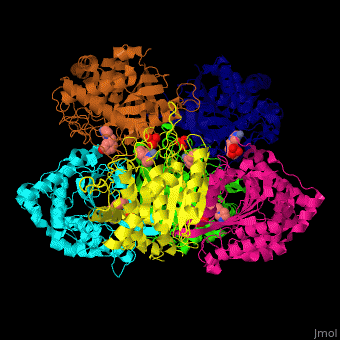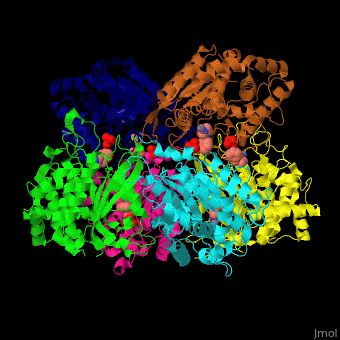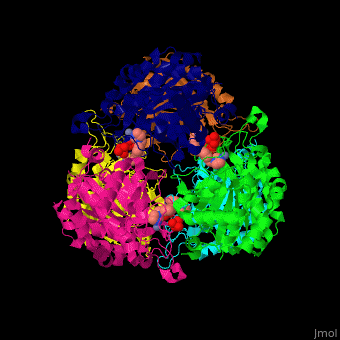
Glutaminyl cyclase
From Proteopedia
(Difference between revisions)
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Structure of human glutaminyl cyclase complex with benzyimidazole and Zn+2 ion (PDB code [[2afx]]).' scene='59/596314/Cv/1'> |
== Function == | == Function == | ||
| - | '''Glutaminyl cyclase''' (QC) catalyzes the conversion of glutaminyl-peptide to 5-oxoprolyl-peptide and ammonia. Thus QC catalyzes the cyclization of an N-terminal glutamyl residue<ref>PMID:3597387</ref>. | + | '''Glutaminyl cyclase''' (QC) or '''glutaminyl-peptide cyclotransferase''' catalyzes the conversion of glutaminyl-peptide to 5-oxoprolyl-peptide and ammonia. Thus QC catalyzes the cyclization of an N-terminal glutamyl residue<ref>PMID:3597387</ref>. |
== Relevance == | == Relevance == | ||
| - | The biological assembly of Human Glutaminyl cyclase is <scene name='59/596314/Cv/ | + | The biological assembly of Human Glutaminyl cyclase is <scene name='59/596314/Cv/6'>homohexamer</scene>. QC is considered as a marker of thyroid tumors. QC acts on the generation of N-terminal pyroglutamyl groups of peptide hormones and amyloid-related plaque-forming peptides. The latter contributes to Alzheimer disease. Alzheimer patients show increased expression of QC<ref>PMID:23207485</ref> and QC inhibitors reduces the disease pathology and symptoms. |
== Structural highlights == | == Structural highlights == | ||
| - | The active site of QC contains a Zn+2 ion<ref>PMID:16135565</ref>. | + | The <scene name='59/596314/Cv/7'>active site of QC contains a Zn+2 ion</scene><ref>PMID:16135565</ref>. |
</StructureSection> | </StructureSection> | ||
| Line 21: | Line 21: | ||
*Glutaminyl cyclase | *Glutaminyl cyclase | ||
| - | **[[2afm]], [[2afo]] – hQC – human <br /> | + | **[[2afm]], [[2afo]] – hQC + Zn – human <br /> |
| + | **[[2afs]], [[2zed]], [[2zee]], [[2zef]], [[2zeg]], [[2zeh]], [[2zel]], [[2zem]], [[2zen]], [[2zeo]], [[2zep]], [[3pbe]], [[4yu9]], [[7cp0]] – hQC (mutant) + Zn<br /> | ||
| + | **[[9fxh]], [[9fxv]] – hQC (mutant) + Co<br /> | ||
| + | **[[9fxg]] – hQC (mutant) <br /> | ||
| + | **[[3pb4]], [[3pb6]] – hQC catalytic domain + Zn<br /> | ||
| + | **[[3si1]] – mQC + Zn – mouse <br /> | ||
| + | **[[4f9u]], [[4fai]], [[4fwu]] – DmQC – ''Drosophila melanogaster'' <br /> | ||
| + | **[[4f9v]], [[4fbe]] – DmQC (mutant) <br /> | ||
| + | **[[2faw]] – QC – papaya<br /> | ||
| + | **[[3mbr]] – QC – ''Xanthomonas campestris'' <br /> | ||
| + | **[[3nol]], [[3nom]] – QC – ''Zymomonas mobilis'' <br /> | ||
**[[3nok]] – QC – ''Myxococcus xanthus''<br /> | **[[3nok]] – QC – ''Myxococcus xanthus''<br /> | ||
| - | **[[ | + | **[[4mhn]], [[4mhp]], [[7d1n]], [[7d1p]], [[7d1y]], [[7d21]], [[7d23]], [[7d2b]], [[7d2d]], [[7d2i]], [[7d2j]] – IsQC catalytic domain – ''Ixodes scapularis''<br /> |
| - | **[[ | + | **[[7d1h]] – IsQC catalytic domain (mutant) <br /> |
| + | **[[7d17]] – QC – ''Macrostormum lignano''<br /> | ||
| + | **[[6qql]], [[7cje]], [[7cjg]] – QC – ''Porphyromonas gingivalis''<br /> | ||
| + | **[[7d18]] – QC – ''Acidobacteriales bacterium''<br /> | ||
| + | **[[7d1b]] – QC – ''Fimbrilglobus ruber''<br /> | ||
| + | **[[6qro]] – QC – ''Tannerella forsythia''<br /> | ||
| + | **[[8vpl]], [[8vpm]] – QC + Zn – ''Aspergillus flavus'' <br /> | ||
*Glutaminyl cyclase complex | *Glutaminyl cyclase complex | ||
| Line 30: | Line 46: | ||
**[[2afu]] – hQC + glutamine t-butyl ester <br /> | **[[2afu]] – hQC + glutamine t-butyl ester <br /> | ||
**[[2afw]] – hQC + N-acetylhistamine <br /> | **[[2afw]] – hQC + N-acetylhistamine <br /> | ||
| - | **[[2afx]], [[3si0]], [[2afz]] – hQC + imidazole derivative<br /> | + | **[[2afx]], [[3si0]], [[2afz]], [[3pb9]], [[7cm0]], [[7d8e]], [[8hy3]], [[8xfv]], [[8xga]], [[8xgb]], [[8xgt]], [[8xgy]], [[9isd]], [[9ivv]], [[9kmb]] – hQC catalytic domain + imidazole derivative + Zn<br /> |
| - | **[[3pbb]] – hQC + inhibitor <br /> | + | **[[9fxi]] – hQC (mutant) + inhibitor + Co<br /> |
| + | **[[3pb7]], [[3pb8]], [[3pbb]] – hQC catalytic domain + inhibitor <br /> | ||
| + | **[[4ywy]], [[6gbx]], [[7coz]] – hQC (mutant) + inhibitor <br /> | ||
| + | **[[6yi1]] – hQC + Glu-(γ-hydrazide)-Phe-Ala<br /> | ||
| + | **[[6yjy]] – hQC + neurotensin<br /> | ||
**[[3si2]] – mQC + inhibitor <br /> | **[[3si2]] – mQC + inhibitor <br /> | ||
| + | **[[7d1d]] – BtQC + imidazole derivative – ''Bacterioides thetaiotaomicron''<br /> | ||
| + | **[[7d1e]] – BtQC + N-acetylhistamine <br /> | ||
| + | **[[4mhz]] – IsQC catalytic domain (mutant) + inhibitor <br /> | ||
| + | **[[4mhy]] – IsQC catalytic domain + imidazole derivative<br /> | ||
}} | }} | ||
Current revision
| |||||||||||
3D Structures of glutaminyl cyclase
Updated on 17-July-2025
References
- ↑ Busby WH Jr, Quackenbush GE, Humm J, Youngblood WW, Kizer JS. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem. 1987 Jun 25;262(18):8532-6. PMID:3597387
- ↑ Valenti MT, Bolognin S, Zanatta C, Donatelli L, Innamorati G, Pampanin M, Zanusso G, Zatta P, Dalle Carbonare L. Increased glutaminyl cyclase expression in peripheral blood of Alzheimer's disease patients. J Alzheimers Dis. 2013;34(1):263-71. doi: 10.3233/JAD-120517. PMID:23207485 doi:http://dx.doi.org/10.3233/JAD-120517
- ↑ Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH. Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13117-22. Epub 2005 Aug 31. PMID:16135565