We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Aconitase
From Proteopedia
(Difference between revisions)
(test morphing) |
|||
| Line 7: | Line 7: | ||
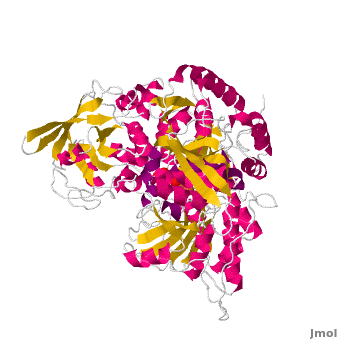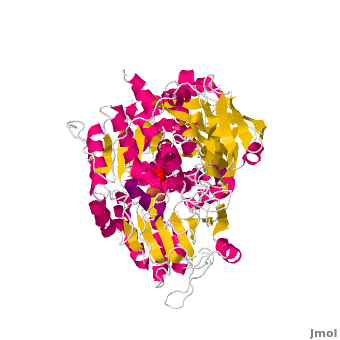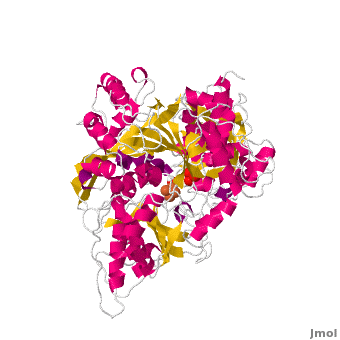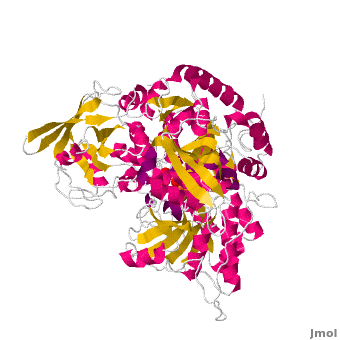
<applet load='Morph_2ipy-2b3x.pdb.gz' scene='User:Ralf_Stephan/Sandbox_1/Morphtest/2' size='400' frame='true' align='right' caption="" />A specialty of cAc is that in mammals it has developed a second function as inhibitor of those mRNA that carry an iron-regulatory element (IRE). Therefore, the cytosolic cAc is named IREBP for IRE-binding protein when this function is talked about. Only one of the two functions is active, depending on whether the (4Fe4S) cofactor is present in the molecule: it's essential for the ACO function. You can see, by looking at the morph, how much the enzyme structure differs between those two functions. | <applet load='Morph_2ipy-2b3x.pdb.gz' scene='User:Ralf_Stephan/Sandbox_1/Morphtest/2' size='400' frame='true' align='right' caption="" />A specialty of cAc is that in mammals it has developed a second function as inhibitor of those mRNA that carry an iron-regulatory element (IRE). Therefore, the cytosolic cAc is named IREBP for IRE-binding protein when this function is talked about. Only one of the two functions is active, depending on whether the (4Fe4S) cofactor is present in the molecule: it's essential for the ACO function. You can see, by looking at the morph, how much the enzyme structure differs between those two functions. | ||
| - | == Available structures == | + | <!--== Available structures == |
In the PDB, nearly all deposited structures are from mammals, [[1l5j]] is from ''E.coli''. Also, only [[2ipy]] shows the IREBP function of cAc---it's also the only from rabbit. There are only two other cAc structures, with and without citrate, also the only from human. All other structures are either cow or pig, and a mutant from pig; all three proteins with several different ligands and inhibitors. | In the PDB, nearly all deposited structures are from mammals, [[1l5j]] is from ''E.coli''. Also, only [[2ipy]] shows the IREBP function of cAc---it's also the only from rabbit. There are only two other cAc structures, with and without citrate, also the only from human. All other structures are either cow or pig, and a mutant from pig; all three proteins with several different ligands and inhibitors. | ||
| Line 29: | Line 29: | ||
*[[7acn]] - mAc (''Sus scrofa'') with isocitrate | *[[7acn]] - mAc (''Sus scrofa'') with isocitrate | ||
*[[8acn]] - mAc (''Sus scrofa'') with nitroisocitrate | *[[8acn]] - mAc (''Sus scrofa'') with nitroisocitrate | ||
| - | + | --> | |
== Weblinks == | == Weblinks == | ||
*[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb89_1.html Molecule of the Month: Aconitase and Iron Regulatory Protein 1] | *[http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb89_1.html Molecule of the Month: Aconitase and Iron Regulatory Protein 1] | ||
*[http://en.wikipedia.org/wiki/Aconitase Aconitase at Wikipedia] | *[http://en.wikipedia.org/wiki/Aconitase Aconitase at Wikipedia] | ||
Revision as of 09:30, 18 February 2009
Aconitase (ACO) is an enzymatic domain that confers the ability to catalyse the equilibrium
- citrate = aconitate + H2O = isocitrate
This reaction is part of the citrate (TCA-, Krebs-)cycle.
In most organims, there is a cytosolic enzyme with an ACO domain (cAc), and in eukaryotes, a second copy of it was introduced with mitochondria (mAc). Plants developed even more copies in mitochondria.
|
Weblinks
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Ralf Stephan, David Canner, Joel L. Sussman, Jaime Prilusky, Anthony Noles, Angel Herraez, Eran Hodis