Prolyl Endopeptidase
From Proteopedia
(Difference between revisions)
(→Inhibition) |
|||
| (29 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='' size='400' side='right' caption='Prolyl endopeptidase complex with Pro-(decarboxy-Pro)-type inhibitor (PDB entry [[3eq7]])' scene='38/382958/Cv/1'> |
| - | Prolyl endopeptidases (PEPs) are a class of serine proteases which cleave peptide bonds on the C-terminal side of internal proline residues.<ref name="Shan2">PMID:15738423</ref> | + | |
| + | '''Prolyl endopeptidases''' (PEPs) are a class of serine proteases which cleave peptide bonds on the C-terminal side of internal proline residues.<ref name="Shan2">PMID:15738423</ref> | ||
== Structure == | == Structure == | ||
| - | [[Image:1yr2_ribbon2.png|thumb|300x300px|alt=Alt text|Catalytic(bottom) and β-propeller domains(top) with peptide linkages between the domains shown in green and blue]] | + | [[Image:1yr2_ribbon2.png|thumb|left|300x300px|alt=Alt text|Catalytic(bottom) and β-propeller domains(top) with peptide linkages between the domains shown in green and blue]] |
| + | {{Clear}} | ||
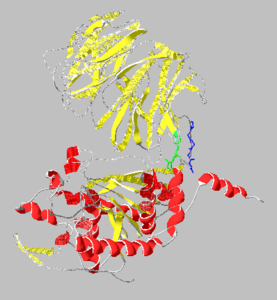
Prolyl endopeptidases are relatively large enzymes(75 kDa) and contain two distinct domains: a catalytic domain and a β-propeller domain<ref name="Besedin">PMID:12658988</ref>. | Prolyl endopeptidases are relatively large enzymes(75 kDa) and contain two distinct domains: a catalytic domain and a β-propeller domain<ref name="Besedin">PMID:12658988</ref>. | ||
| Line 13: | Line 15: | ||
The catalytic domain mainly consists of an [http://en.wikipedia.org/wiki/Alpha/beta_hydrolase_fold α/β hydrolase fold] which is a series of 8 strands connected by helices in a fold common to many enzymes. The catalytic domain was found to be much more conserved than the β-propeller domain(~50%).<ref name="Gass">PMID:17160352</ref> The catalytic site contains a catalytic triad of Ser-Asp-His and lies in a cavity near the interface to the β-propeller domain.<ref name="Shan2"/> | The catalytic domain mainly consists of an [http://en.wikipedia.org/wiki/Alpha/beta_hydrolase_fold α/β hydrolase fold] which is a series of 8 strands connected by helices in a fold common to many enzymes. The catalytic domain was found to be much more conserved than the β-propeller domain(~50%).<ref name="Gass">PMID:17160352</ref> The catalytic site contains a catalytic triad of Ser-Asp-His and lies in a cavity near the interface to the β-propeller domain.<ref name="Shan2"/> | ||
| - | + | == Binding Mechanism == | |
From observed interactions between the β-propeller and catalytic domains several possible substrate binding mechanism have been proposed. | From observed interactions between the β-propeller and catalytic domains several possible substrate binding mechanism have been proposed. | ||
| Line 22: | Line 24: | ||
Both mechanistic binding theories account for the observed activity of PEP as being limited to acting on small peptides of less than 30 amino acids. | Both mechanistic binding theories account for the observed activity of PEP as being limited to acting on small peptides of less than 30 amino acids. | ||
| - | + | == Inhibition == | |
Initial classification of prolyl endopeptidases as [http://en.wikipedia.org/wiki/Serine_protease serine proteases] was due to their inhibition by [http://en.wikipedia.org/wiki/Diisopropyl_fluorophosphate DFP].<ref name="Besedin"/> | Initial classification of prolyl endopeptidases as [http://en.wikipedia.org/wiki/Serine_protease serine proteases] was due to their inhibition by [http://en.wikipedia.org/wiki/Diisopropyl_fluorophosphate DFP].<ref name="Besedin"/> | ||
| Line 35: | Line 37: | ||
== Pharmaceutical Possibilities == | == Pharmaceutical Possibilities == | ||
| - | Both human and microbial PEP have been the focus of research into their viability as therapeutic agents for several diseases. The ability of PEPs to cleave internal proline peptide bonds is of interest in the treatment of Celiac Disease which is caused by a reaction to gluten, a proline rich protein found in wheat and wheat subspecies.<ref name="Besedin"/> PEPs have also been linked to several neurological disorders due to high activity in the brain and proposed role in the degradation of neuropeptides.<ref name="Gass"/> | + | Both human and microbial PEP have been the focus of research into their viability as therapeutic agents for several diseases. The ability of PEPs to cleave internal proline peptide bonds is of interest in the treatment of [http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease] which is caused by a reaction to gluten, a proline rich protein found in wheat and wheat subspecies.<ref name="Besedin"/> PEPs have also been linked to several neurological disorders due to high activity in the brain and a proposed role in the degradation of neuropeptides.<ref name="Gass"/> |
=== Celiac Disease === | === Celiac Disease === | ||
| Line 41: | Line 43: | ||
[http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease](CD) is a genetic disorder marked by diarrhea, fatigue, weight loss, and villous atrophy due to the consumption of certain proteins such as gluten and other [http://en.wikipedia.org/wiki/Prolamin prolamins] found in wheat and wheat subspecies. The symptoms of CD are due to an auto-immune inflammatory reaction that occurs in the small intestine due to [http://en.wikipedia.org/wiki/Prolamin prolamins] that are rich in proline residues and known to be resistant to many proteases. | [http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease](CD) is a genetic disorder marked by diarrhea, fatigue, weight loss, and villous atrophy due to the consumption of certain proteins such as gluten and other [http://en.wikipedia.org/wiki/Prolamin prolamins] found in wheat and wheat subspecies. The symptoms of CD are due to an auto-immune inflammatory reaction that occurs in the small intestine due to [http://en.wikipedia.org/wiki/Prolamin prolamins] that are rich in proline residues and known to be resistant to many proteases. | ||
| - | There is currently no cure for [http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease] and the main treatment is to simply avoid eating [http://en.wikipedia.org/wiki/Prolamin | + | There is currently no cure for [http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease] and the main treatment is to simply avoid eating foods containing [http://en.wikipedia.org/wiki/Prolamin prolamins]. |
| - | Prolyl endopeptidases are a promising therapeutic agent due to their ability to degrade | + | Prolyl endopeptidases are a promising therapeutic agent due to their ability to degrade proline rich [http://en.wikipedia.org/wiki/Prolamin prolamins] and inhibit the inflammatory reaction. An early idea for treatment is to orally administer certain microbial PEPs which could directly degrade [http://en.wikipedia.org/wiki/Prolamin prolamins] in the intestine and reduce the auto-immune response. The key for this therapy would be to to protect the PEPs from being degraded by gastric acids while allowing them to be able to be functional in the small intestine once the acidity is decreased.<ref name="Ehren2">PMID:19621078</ref> |
| - | Research by Ehren, Govindarajan, Morón, Minshull, and Khosla has shown the ability to engineer the prolyl endopeptidase of ''Sphingomonas capsulata'' to increase the activity of this PEP under simulated gastric conditions showing the relevance of PEPs as a potential therapeutic agent for [http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease].<ref name="Ehren1">PMID: | + | Research by Ehren, Govindarajan, Morón, Minshull, and Khosla has shown the ability to engineer the prolyl endopeptidase of ''Sphingomonas capsulata'' to increase the activity of this PEP under simulated gastric conditions showing the relevance of PEPs as a potential therapeutic agent for [http://en.wikipedia.org/wiki/Coeliac_disease Celiac Disease].<ref name="Ehren1">PMID:18836204</ref> |
=== Neurological Disorders === | === Neurological Disorders === | ||
| - | As well as having a proposed role in the degradation of neuropeptides PEPs have also been linked to several specific neurological disorders. It has been found that patients suffering from depression had decreased PEP activity and those suffering from psychosis have increased PEP activity showing the importance of maintaining a balanced level of PEP activity in the brain. PEP inhibitors are a current point of research in hopes of finding ways to balance brain PEP activity. | + | As well as having a proposed role in the degradation of neuropeptides PEPs have also been linked to several specific neurological disorders. It has been found that patients suffering from depression had decreased PEP activity and those suffering from psychosis have increased PEP activity showing the importance of maintaining a balanced level of PEP activity in the brain. PEP inhibitors are a current point of research in hopes of finding ways to balance brain PEP activity.<ref name="Gass"/> |
| + | |||
| + | == Structural highlights == | ||
| + | The <scene name='38/382958/Cv/4'>active site of PEP contains the serine protease catalytic triad of Ser-His-Asp</scene><ref>PMID:19006380</ref>. Triad is colored in salmon. | ||
| + | |||
| + | ==3D structures of prolyl endopeptidase== | ||
| + | |||
| + | [[Prolyl endopeptidase 3D structures]] | ||
==References== | ==References== | ||
<References/> | <References/> | ||
| + | </StructureSection> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Stacey Shantz, Michal Harel, Alexander Berchansky, Joel L. Sussman, Andrea Gorrell, David Canner