We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sonic Hedgehog
From Proteopedia
(Difference between revisions)
| (35 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
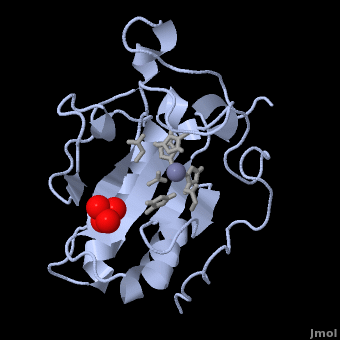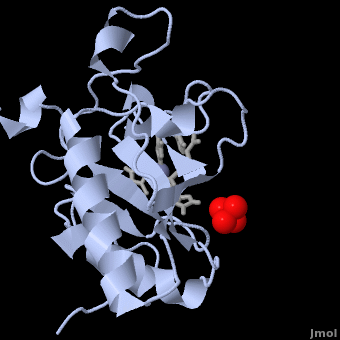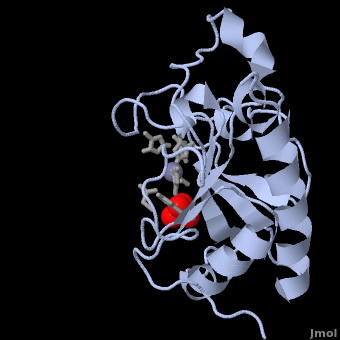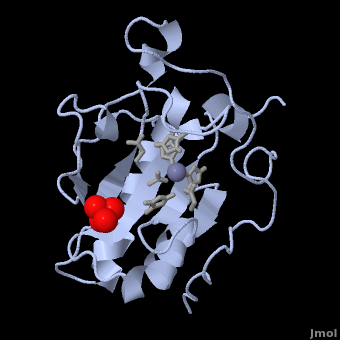
| - | < | + | <StructureSection load='' size='350' side='right' scene='Sandbox_191/Scenedefault/4' caption='Mouse sonic hedgehog complex with sulfate and Zn+2 ion (grey) (PDB code [[1vhh]])'> |
| - | '''SONIC HEDGEHOG''' | ||
| - | {{STRUCTURE_1vhh | PDB=1vhh | SCENE=Sandbox_191/Scenedefault/4}} | ||
= Introduction = | = Introduction = | ||
| - | Sonic hedgehog (Shh) is a member of the Hedgehog (Hh) family of secreted extracellular signaling proteins, which serve important roles in regulating both short-range and long-range patterning processes in developing invertebrate and vertebrate tissues<ref>PMID: 7867057</ref>. First discovered in ''Drosophila'', where mutations of the single ''Hedgehog'' gene produces larvae that are covered in hedgehog-like denticles, Hh proteins are encoded by at least three genes in mammals - ''Sonic'', ''Desert'', and ''Indian hedgehog''<ref>PMID: 7916661</ref>. With the ability to control such fundamental processes as the anterioposterior patterning of vertebrate limb buds<ref>PMID: 8269518</ref>, the formation of motor neurons in the neural tube <ref name="neuron">PMID: 7736596</ref>, and the development and maintenance of tissues and organs<ref>PMID: 10980429</ref>, Shh is the most well-studied member of the Hh signaling proteins<ref name="papinsky">PMID: 10753901</ref>. Excessive signaling in adult cells has been implicated in the development of several human cancers<ref>PMID: 14737121</ref><ref name="Path">PMID: 12044012</ref>. | + | '''Sonic hedgehog''' (Shh) is a member of the Hedgehog (Hh) family of secreted extracellular signaling proteins, which serve important roles in regulating both short-range and long-range patterning processes in developing invertebrate and vertebrate tissues<ref>PMID: 7867057</ref>. First discovered in ''Drosophila'', where mutations of the single ''Hedgehog'' gene produces larvae that are covered in hedgehog-like denticles, Hh proteins are encoded by at least three genes in mammals - ''Sonic'', ''Desert'', and ''Indian hedgehog''<ref>PMID: 7916661</ref>. With the ability to control such fundamental processes as the anterioposterior patterning of vertebrate limb buds<ref name="limb">PMID: 8269518</ref>, the formation of motor neurons in the neural tube <ref name="neuron">PMID: 7736596</ref>, and the development and maintenance of tissues and organs<ref>PMID: 10980429</ref>, Shh is the most well-studied member of the Hh signaling proteins<ref name="papinsky">PMID: 10753901</ref>. Excessive signaling in adult cells has been implicated in the development of several human cancers<ref>PMID: 14737121</ref><ref name="Path">PMID: 12044012</ref>. Mutations in the ''Shh'' gene can also result in severe developmental consequences, including [http://en.wikipedia.org/wiki/Holoprosencephaly holoprosencephaly] <ref>PMID: 8896572</ref>. |
| + | |||
| + | See also [[Hedgehog signaling pathway]]. | ||
= Biosynthesis = | = Biosynthesis = | ||
| - | As with all members of the Hh family, Shh biosynthesis begins with an unusual molecular processing event. Following cleavage of its signal peptide, the Shh precursor protein is autocatalytically cleaved into two functionally distinct domains, a 19-kDa amino-terminal domain (Shh-N) and a 27-kDa carboxy-terminal domain (Shh-C)<ref>PMID: 7891723</ref>. Spanning residues 24 to 197 in human Shh, Shh-N is responsible for all of the local and long-range signaling activities of Shh. Shh-C possesses an intramolecular transferase activity responsible for covalent attachment of a molecule of cholesterol to the C-terminus of Shh-N <ref name="papinsky"/>. The addition of cholesterol serves to tether Shh-N to the cell membrane, restricting its range of activity to that of local signaling only<ref>PMID: 8824192</ref>. A second modification involving the attachment of a palmitoyl group to Cys-24 on the protein's N-terminus has recently been discovered in insect and mammalian cells. This N-terminal modification is thought to increase the potency of the Shh-N signal as much as 30-fold<ref>PMID: 9593755</ref>. | + | As with all members of the Hh family, Shh biosynthesis begins with an unusual molecular processing event. Following cleavage of its signal peptide, the Shh precursor protein is autocatalytically cleaved into two functionally distinct domains, a 19-kDa amino-terminal domain (Shh-N) and a 27-kDa carboxy-terminal domain (Shh-C)<ref name="process">PMID: 7891723</ref>. Spanning residues 24 to 197 in human Shh, Shh-N is responsible for all of the local and long-range signaling activities of Shh. Shh-C possesses an intramolecular transferase activity responsible for covalent attachment of a molecule of cholesterol to the C-terminus of Shh-N <ref name="papinsky"/>. The addition of cholesterol serves to tether Shh-N to the cell membrane, restricting its range of activity to that of local signaling only<ref>PMID: 8824192</ref>. A second modification involving the attachment of a palmitoyl group to Cys-24 on the protein's N-terminus has recently been discovered in insect and mammalian cells. This N-terminal modification is thought to increase the potency of the Shh-N signal as much as 30-fold<ref>PMID: 9593755</ref>. |
= Structural Overview = | = Structural Overview = | ||
| Line 15: | Line 15: | ||
[[Image:Catalytic site.png |left| thumb | '''Figure 1.''' A close-up of the zinc coordination site of Shh-N, showing His 141, Asp 148, and His 183 separated by distances of 2.06, 1.97, and 2.08 Å, respectively. The zinc-bound water molecule is also shown in line with Glu 177, which is thought to participate in hydrolysis by abstracting a proton from the water molecule<ref name="Palm"/>.]] The three-dimensional structure of murine Shh-N (residues 39-195) is shown as 1VHH. An α + β sandwich consisting of two <scene name='Sandbox_191/Scene2/5'> α-helices</scene> and a six-stranded, mixed <scene name='Sandbox_191/Scene3/5'> β-sheet</scene> makes up the core of the structure, along with a two-stranded, antiparallel β-sheet<ref name="Palm"/>. Although this type of folding arrangement has not yet been seen in other proteins, the presence of a <scene name='Sandbox_191/Scene4/3'>tetrahedrally coordinated zinc ion</scene> in Shh-N bears close structural resemblance to the zinc coordination sites of zinc hydrolases, including thermolysin and carboxypeptidase A. Three amino acid side chains – <scene name='Sandbox_191/Scene4/4'>His 141, Asp 148, and His 183</scene> – are bound to the zinc ion in the crystal structure, along with a single <scene name='Sandbox_191/Scene4/5'>molecule of water</scene> (Figure 1). Zinc ions that serve a structural role in proteins are normally coordinated by four amino acid side chains and are not usually exposed to the surrounding solvent. The presence of a zinc-bound water molecule in Shh-N, by contrast, is indicative of a catalytic function. In zinc hydrolases, the water molecule is key to the protein's enzymatic activity when its proton is removed by a nearby glutamate residue. <scene name='Sandbox_191/Scene4/6'>Glu 177</scene> (Figure 1) likely serves the same role in Shh-N, further supporting a novel, hydrolytic function for this protein. Based on the catalytic mechanisms for thermolysin and carboxypeptidase A, three non-coordinating residues in Shh-N (<scene name='Sandbox_191/Scene4/7'>His 135, His 181, and Glu 127</scene>) are also believed to participate in a potential hydrolysis reaction<ref name="Palm">PMID: 7477329</ref>. | [[Image:Catalytic site.png |left| thumb | '''Figure 1.''' A close-up of the zinc coordination site of Shh-N, showing His 141, Asp 148, and His 183 separated by distances of 2.06, 1.97, and 2.08 Å, respectively. The zinc-bound water molecule is also shown in line with Glu 177, which is thought to participate in hydrolysis by abstracting a proton from the water molecule<ref name="Palm"/>.]] The three-dimensional structure of murine Shh-N (residues 39-195) is shown as 1VHH. An α + β sandwich consisting of two <scene name='Sandbox_191/Scene2/5'> α-helices</scene> and a six-stranded, mixed <scene name='Sandbox_191/Scene3/5'> β-sheet</scene> makes up the core of the structure, along with a two-stranded, antiparallel β-sheet<ref name="Palm"/>. Although this type of folding arrangement has not yet been seen in other proteins, the presence of a <scene name='Sandbox_191/Scene4/3'>tetrahedrally coordinated zinc ion</scene> in Shh-N bears close structural resemblance to the zinc coordination sites of zinc hydrolases, including thermolysin and carboxypeptidase A. Three amino acid side chains – <scene name='Sandbox_191/Scene4/4'>His 141, Asp 148, and His 183</scene> – are bound to the zinc ion in the crystal structure, along with a single <scene name='Sandbox_191/Scene4/5'>molecule of water</scene> (Figure 1). Zinc ions that serve a structural role in proteins are normally coordinated by four amino acid side chains and are not usually exposed to the surrounding solvent. The presence of a zinc-bound water molecule in Shh-N, by contrast, is indicative of a catalytic function. In zinc hydrolases, the water molecule is key to the protein's enzymatic activity when its proton is removed by a nearby glutamate residue. <scene name='Sandbox_191/Scene4/6'>Glu 177</scene> (Figure 1) likely serves the same role in Shh-N, further supporting a novel, hydrolytic function for this protein. Based on the catalytic mechanisms for thermolysin and carboxypeptidase A, three non-coordinating residues in Shh-N (<scene name='Sandbox_191/Scene4/7'>His 135, His 181, and Glu 127</scene>) are also believed to participate in a potential hydrolysis reaction<ref name="Palm">PMID: 7477329</ref>. | ||
| - | The crystal structure of Shh-N contains a single sulphate molecule. | + | |
| + | The crystal structure of Shh-N also contains a single sulphate molecule, shown in red and yellow, which interacts with arginine residues at the interface of two Shh-N molecules. Since Shh-N binds [http://en.wikipedia.org/wiki/Heparin heparin], this interaction is believed to comprise a heparin-binding region<ref name="Palm"/>. | ||
| + | |||
= Function = | = Function = | ||
| - | [[Image: Short and Long-Range.jpg | thumb | '''Figure 2.''' Shh-N is released from the cell membrane for long-range signaling by zinc-dependent proteolysis <ref name="signal"/>. ]]The tetrahedrally coordinated zinc ion of Shh-N, along with the non-coordinating residues thought to assist hydrolysis, are highly conserved among vertebrate Hh proteins. A potential hydrolytic activity is therefore expected to play an important cellular role. In pursuit of a substrate for Shh-N, it was found that <scene name='Sandbox_191/Scene3/6'>Ala 194 and Lys 195</scene> near the C-terminus of one Shh-N molecule can hydrogen bond with residues in the zinc binding site of a second Shh-N molecule. This indicates that the protein may be capable of cleaving between Lys 195 and Ser 196 within its own C-terminus <ref name="Palm"/>. This is the most highly conserved region of Hh proteins<ref name="signal">PMID: 8807822</ref>. The suspected autoproteolytic function of Shh-N has been suggested to liberate the tethered protein from the cell membrane to facilitate long-range signaling <ref name="Palm"/>. However, other possible substrates for Shh-N are also likely, including an Shh receptor or other types of signaling molecules involved in the Shh pathway. Whichever the substrate, the discovery of a potential proteolytic activity for Shh-N seems to provide a mechanism for regulating short-range and long-range signaling, which until now has been poorly understood<ref name="papinsky"/>. Short-range signaling occurs in a contact-dependent fashion and is associated with induction of the floor plate within the neural tube. During long-range signaling, Shh-N acts as a morphogen to establish somite patterning, motor neuron formation in the neural tube<ref name="neuron"/>, and anteroposterior limb patterning. | + | [[Image: Short and Long-Range.jpg | thumb | '''Figure 2.''' Shh-N is released from the cell membrane for long-range signaling by zinc-dependent proteolysis. [Note: This figure is adapted from references <ref name="Palm"/> and <ref name="signal"/>.] ]]The tetrahedrally coordinated zinc ion of Shh-N, along with the non-coordinating residues thought to assist hydrolysis, are highly conserved among vertebrate Hh proteins. A potential hydrolytic activity is therefore expected to play an important cellular role. In pursuit of a substrate for Shh-N, it was found that <scene name='Sandbox_191/Scene3/6'>Ala 194 and Lys 195</scene> near the C-terminus of one Shh-N molecule can hydrogen bond with residues in the zinc binding site of a second Shh-N molecule. This indicates that the protein may be capable of cleaving between Lys 195 and Ser 196 within its own C-terminus <ref name="Palm"/>. This is the most highly conserved region of Hh proteins<ref name="signal">PMID: 8807822</ref>. The suspected autoproteolytic function of Shh-N has been suggested to liberate the tethered protein from the cell membrane to facilitate long-range signaling (Figure 2)<ref name="Palm"/>. However, other possible substrates for Shh-N are also likely, including an Shh receptor or other types of signaling molecules involved in the Shh pathway. Whichever the substrate, the discovery of a potential proteolytic activity for Shh-N seems to provide a mechanism for regulating short-range and long-range signaling, which until now has been poorly understood<ref name="papinsky"/>. Short-range signaling occurs in a contact-dependent fashion and is associated with induction of the [http://en.wikipedia.org/wiki/Floor_plate floor plate] within the neural tube<ref>PMID: 8223247</ref>. During long-range signaling, Shh-N acts as a morphogen to establish somite patterning<ref name="process"/>, motor neuron formation in the neural tube<ref name="neuron"/>, and anteroposterior limb patterning <ref name="limb"/>. |
== Sonic Signaling: The Shh-Gli Pathway == | == Sonic Signaling: The Shh-Gli Pathway == | ||
| - | [[Image: SHH SIGNALING PATHWAY.jpg |left| thumb| '''Figure 3.''' The Sonic hedgehog signaling pathway. In the absence of Shh, Patched inhibits Smo. Inhibition of Patched by Shh activates normal developmental processes. [Note: This figure is adapted from references <ref name="Path"/> and <ref name="ShhGli">PMID: 16339192</ref>.] ]] In the absence of a Shh signal, a 12 transmembrane receptor protein called Patched blocks the function of Smoothened (Smo), a seven-pass transmembrane protein, by keeping it sequestered in an intracellular vesicle (Figure 3)<ref name="ShhGli"/>. When Shh binds to Patched, inhibition of Smo by Patched is relieved. Patched becomes endocytosed, and Smo translocates to the cell surface. In vertebrates, Smo localizes to the surface of the primary cilium, initiating a signaling cascade that leads to the activation of Gli transcription factors <ref name="Path"/>. Present in both the nucleus and cytoplasm, there are three of these regulatory proteins (''Gli1'', ''Gli2'', and ''Gli3''). Following Shh signaling, all three proteins can act as transcriptional activators of Shh target genes. Gli3, however, can act as both an activator and repressor; in the absence of Shh signaling, Gli3 is cleaved by the proteasome, and its truncated form accumulates in the nucleus where it represses transcription of Shh-responsive genes (Figure 3) <ref name="ShhGli"/>. | + | [[Image: SHH SIGNALING PATHWAY.jpg |left| thumb| '''Figure 3.''' The Sonic hedgehog signaling pathway. In the absence of Shh, Patched inhibits Smo. Inhibition of Patched by Shh activates normal developmental processes. [Note: This figure is adapted from references <ref name="Path"/> and <ref name="ShhGli">PMID: 16339192</ref>.] ]] |
| - | + | {{Clear}} | |
| - | = | + | In the absence of a Shh signal, a 12 transmembrane receptor protein called Patched blocks the function of Smoothened (Smo), a seven-pass transmembrane protein, by keeping it sequestered in an intracellular vesicle (Figure 3)<ref name="ShhGli"/>. When Shh binds to Patched, inhibition of Smo by Patched is relieved. Patched becomes endocytosed, and Smo translocates to the cell surface. In vertebrates, Smo localizes to the surface of the primary cilium, initiating a signaling cascade that leads to the activation of Gli transcription factors <ref name="Path"/>. Present in both the nucleus and cytoplasm, there are three of these regulatory proteins (''Gli1'', ''Gli2'', and ''Gli3''). Following Shh signaling, all three proteins can act as transcriptional activators of Shh target genes. Gli3, however, can act as both an activator and repressor; in the absence of Shh signaling, Gli3 is cleaved by the proteasome, and its truncated form accumulates in the nucleus where it represses transcription of Shh-responsive genes (Figure 3) <ref name="ShhGli"/>. |
| + | = 3D Structures of sonic hedgehog = | ||
| + | [[Sonic hedgehog 3D structures]] | ||
| + | =Additional Resources= | ||
| + | For additional information, See: [[Cancer]] <br /> | ||
| + | </StructureSection> | ||
| + | |||
| + | =References= | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Perrimon N. Hedgehog and beyond. Cell. 1995 Feb 24;80(4):517-20. PMID:7867057
- ↑ Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993 Dec 31;75(7):1417-30. PMID:7916661
- ↑ 3.0 3.1 Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993 Dec 31;75(7):1401-16. PMID:8269518
- ↑ 4.0 4.1 Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995 May 5;81(3):445-55. PMID:7736596
- ↑ Chuang PT, Kornberg TB. On the range of hedgehog signaling. Curr Opin Genet Dev. 2000 Oct;10(5):515-22. PMID:10980429
- ↑ 6.0 6.1 6.2 Pepinsky RB, Rayhorn P, Day ES, Dergay A, Williams KP, Galdes A, Taylor FR, Boriack-Sjodin PA, Garber EA. Mapping sonic hedgehog-receptor interactions by steric interference. J Biol Chem. 2000 Apr 14;275(15):10995-1001. PMID:10753901
- ↑ Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003 Dec;3(12):903-11. PMID:14737121 doi:10.1038/nrc1229
- ↑ 8.0 8.1 8.2 Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002 May;2(5):361-72. PMID:12044012 doi:10.1038/nrc796
- ↑ Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996 Nov;14(3):357-60. PMID:8896572 doi:10.1038/ng1196-357
- ↑ 10.0 10.1 Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol. 1995 Apr;15(4):2294-303. PMID:7891723
- ↑ Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996 Oct 11;274(5285):255-9. PMID:8824192
- ↑ Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998 May 29;273(22):14037-45. PMID:9593755
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Hall TM, Porter JA, Beachy PA, Leahy DJ. A potential catalytic site revealed by the 1.7-A crystal structure of the amino-terminal signalling domain of Sonic hedgehog. Nature. 1995 Nov 9;378(6553):212-6. PMID:7477329 doi:http://dx.doi.org/10.1038/378212a0
- ↑ 14.0 14.1 Bumcrot DA, McMahon AP. Sonic hedgehog: making the gradient. Chem Biol. 1996 Jan;3(1):13-6. PMID:8807822
- ↑ Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205-18. PMID:8223247
- ↑ 16.0 16.1 16.2 Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006 Jan;133(1):3-14. PMID:16339192 doi:133/1/3
Proteopedia Page Contributors and Editors (what is this?)
Randi Woodbeck, Michal Harel, Joel L. Sussman, David Canner, Riley Hicks, Andrea Gorrell, Alexander Berchansky
![Figure 2. Shh-N is released from the cell membrane for long-range signaling by zinc-dependent proteolysis. [Note: This figure is adapted from references and .]](/wiki/images/thumb/4/46/Short_and_Long-Range.jpg/180px-Short_and_Long-Range.jpg)
![Figure 3. The Sonic hedgehog signaling pathway. In the absence of Shh, Patched inhibits Smo. Inhibition of Patched by Shh activates normal developmental processes. [Note: This figure is adapted from references and .]](/wiki/images/thumb/8/85/SHH_SIGNALING_PATHWAY.jpg/180px-SHH_SIGNALING_PATHWAY.jpg)
