Sonic Hedgehog
From Proteopedia
(Difference between revisions)
| (19 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
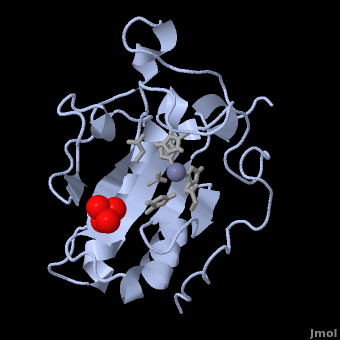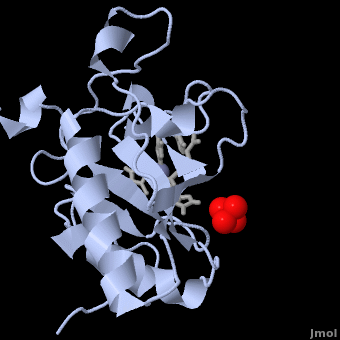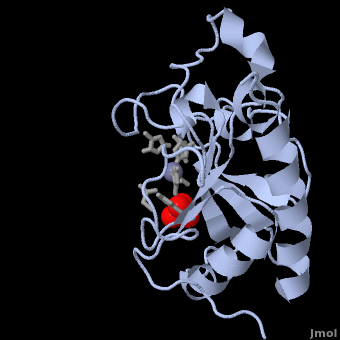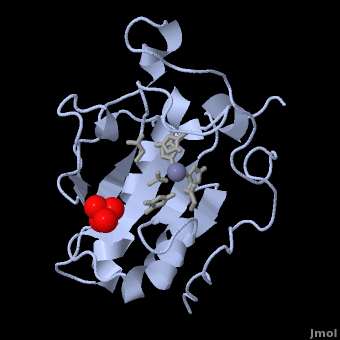
| - | < | + | <StructureSection load='' size='350' side='right' scene='Sandbox_191/Scenedefault/4' caption='Mouse sonic hedgehog complex with sulfate and Zn+2 ion (grey) (PDB code [[1vhh]])'> |
| - | '''SONIC HEDGEHOG''' | ||
| - | {{STRUCTURE_1vhh | PDB=1vhh | SCENE=Sandbox_191/Scenedefault/4}} | ||
= Introduction = | = Introduction = | ||
| - | Sonic hedgehog (Shh) is a member of the Hedgehog (Hh) family of secreted extracellular signaling proteins, which serve important roles in regulating both short-range and long-range patterning processes in developing invertebrate and vertebrate tissues<ref>PMID: 7867057</ref>. First discovered in ''Drosophila'', where mutations of the single ''Hedgehog'' gene produces larvae that are covered in hedgehog-like denticles, Hh proteins are encoded by at least three genes in mammals - ''Sonic'', ''Desert'', and ''Indian hedgehog''<ref>PMID: 7916661</ref>. With the ability to control such fundamental processes as the anterioposterior patterning of vertebrate limb buds<ref name="limb">PMID: 8269518</ref>, the formation of motor neurons in the neural tube <ref name="neuron">PMID: 7736596</ref>, and the development and maintenance of tissues and organs<ref>PMID: 10980429</ref>, Shh is the most well-studied member of the Hh signaling proteins<ref name="papinsky">PMID: 10753901</ref>. Excessive signaling in adult cells has been implicated in the development of several human cancers<ref>PMID: 14737121</ref><ref name="Path">PMID: 12044012</ref>. Mutations in the ''Shh'' gene can also result in severe developmental consequences, including [http://en.wikipedia.org/wiki/Holoprosencephaly holoprosencephaly] <ref>PMID: 8896572</ref>. | + | '''Sonic hedgehog''' (Shh) is a member of the Hedgehog (Hh) family of secreted extracellular signaling proteins, which serve important roles in regulating both short-range and long-range patterning processes in developing invertebrate and vertebrate tissues<ref>PMID: 7867057</ref>. First discovered in ''Drosophila'', where mutations of the single ''Hedgehog'' gene produces larvae that are covered in hedgehog-like denticles, Hh proteins are encoded by at least three genes in mammals - ''Sonic'', ''Desert'', and ''Indian hedgehog''<ref>PMID: 7916661</ref>. With the ability to control such fundamental processes as the anterioposterior patterning of vertebrate limb buds<ref name="limb">PMID: 8269518</ref>, the formation of motor neurons in the neural tube <ref name="neuron">PMID: 7736596</ref>, and the development and maintenance of tissues and organs<ref>PMID: 10980429</ref>, Shh is the most well-studied member of the Hh signaling proteins<ref name="papinsky">PMID: 10753901</ref>. Excessive signaling in adult cells has been implicated in the development of several human cancers<ref>PMID: 14737121</ref><ref name="Path">PMID: 12044012</ref>. Mutations in the ''Shh'' gene can also result in severe developmental consequences, including [http://en.wikipedia.org/wiki/Holoprosencephaly holoprosencephaly] <ref>PMID: 8896572</ref>. |
| + | |||
| + | See also [[Hedgehog signaling pathway]]. | ||
= Biosynthesis = | = Biosynthesis = | ||
| Line 24: | Line 24: | ||
== Sonic Signaling: The Shh-Gli Pathway == | == Sonic Signaling: The Shh-Gli Pathway == | ||
| - | [[Image: SHH SIGNALING PATHWAY.jpg |left| thumb| '''Figure 3.''' The Sonic hedgehog signaling pathway. In the absence of Shh, Patched inhibits Smo. Inhibition of Patched by Shh activates normal developmental processes. [Note: This figure is adapted from references <ref name="Path"/> and <ref name="ShhGli">PMID: 16339192</ref>.] ]] In the absence of a Shh signal, a 12 transmembrane receptor protein called Patched blocks the function of Smoothened (Smo), a seven-pass transmembrane protein, by keeping it sequestered in an intracellular vesicle (Figure 3)<ref name="ShhGli"/>. When Shh binds to Patched, inhibition of Smo by Patched is relieved. Patched becomes endocytosed, and Smo translocates to the cell surface. In vertebrates, Smo localizes to the surface of the primary cilium, initiating a signaling cascade that leads to the activation of Gli transcription factors <ref name="Path"/>. Present in both the nucleus and cytoplasm, there are three of these regulatory proteins (''Gli1'', ''Gli2'', and ''Gli3''). Following Shh signaling, all three proteins can act as transcriptional activators of Shh target genes. Gli3, however, can act as both an activator and repressor; in the absence of Shh signaling, Gli3 is cleaved by the proteasome, and its truncated form accumulates in the nucleus where it represses transcription of Shh-responsive genes (Figure 3) <ref name="ShhGli"/>. | + | [[Image: SHH SIGNALING PATHWAY.jpg |left| thumb| '''Figure 3.''' The Sonic hedgehog signaling pathway. In the absence of Shh, Patched inhibits Smo. Inhibition of Patched by Shh activates normal developmental processes. [Note: This figure is adapted from references <ref name="Path"/> and <ref name="ShhGli">PMID: 16339192</ref>.] ]] |
| - | + | {{Clear}} | |
| + | In the absence of a Shh signal, a 12 transmembrane receptor protein called Patched blocks the function of Smoothened (Smo), a seven-pass transmembrane protein, by keeping it sequestered in an intracellular vesicle (Figure 3)<ref name="ShhGli"/>. When Shh binds to Patched, inhibition of Smo by Patched is relieved. Patched becomes endocytosed, and Smo translocates to the cell surface. In vertebrates, Smo localizes to the surface of the primary cilium, initiating a signaling cascade that leads to the activation of Gli transcription factors <ref name="Path"/>. Present in both the nucleus and cytoplasm, there are three of these regulatory proteins (''Gli1'', ''Gli2'', and ''Gli3''). Following Shh signaling, all three proteins can act as transcriptional activators of Shh target genes. Gli3, however, can act as both an activator and repressor; in the absence of Shh signaling, Gli3 is cleaved by the proteasome, and its truncated form accumulates in the nucleus where it represses transcription of Shh-responsive genes (Figure 3) <ref name="ShhGli"/>. | ||
| + | = 3D Structures of sonic hedgehog = | ||
| + | [[Sonic hedgehog 3D structures]] | ||
| + | =Additional Resources= | ||
| + | For additional information, See: [[Cancer]] <br /> | ||
| + | </StructureSection> | ||
| + | |||
=References= | =References= | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Perrimon N. Hedgehog and beyond. Cell. 1995 Feb 24;80(4):517-20. PMID:7867057
- ↑ Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993 Dec 31;75(7):1417-30. PMID:7916661
- ↑ 3.0 3.1 Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993 Dec 31;75(7):1401-16. PMID:8269518
- ↑ 4.0 4.1 Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995 May 5;81(3):445-55. PMID:7736596
- ↑ Chuang PT, Kornberg TB. On the range of hedgehog signaling. Curr Opin Genet Dev. 2000 Oct;10(5):515-22. PMID:10980429
- ↑ 6.0 6.1 6.2 Pepinsky RB, Rayhorn P, Day ES, Dergay A, Williams KP, Galdes A, Taylor FR, Boriack-Sjodin PA, Garber EA. Mapping sonic hedgehog-receptor interactions by steric interference. J Biol Chem. 2000 Apr 14;275(15):10995-1001. PMID:10753901
- ↑ Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003 Dec;3(12):903-11. PMID:14737121 doi:10.1038/nrc1229
- ↑ 8.0 8.1 8.2 Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002 May;2(5):361-72. PMID:12044012 doi:10.1038/nrc796
- ↑ Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996 Nov;14(3):357-60. PMID:8896572 doi:10.1038/ng1196-357
- ↑ 10.0 10.1 Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol. 1995 Apr;15(4):2294-303. PMID:7891723
- ↑ Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996 Oct 11;274(5285):255-9. PMID:8824192
- ↑ Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998 May 29;273(22):14037-45. PMID:9593755
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Hall TM, Porter JA, Beachy PA, Leahy DJ. A potential catalytic site revealed by the 1.7-A crystal structure of the amino-terminal signalling domain of Sonic hedgehog. Nature. 1995 Nov 9;378(6553):212-6. PMID:7477329 doi:http://dx.doi.org/10.1038/378212a0
- ↑ 14.0 14.1 Bumcrot DA, McMahon AP. Sonic hedgehog: making the gradient. Chem Biol. 1996 Jan;3(1):13-6. PMID:8807822
- ↑ Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205-18. PMID:8223247
- ↑ 16.0 16.1 16.2 Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006 Jan;133(1):3-14. PMID:16339192 doi:133/1/3
Proteopedia Page Contributors and Editors (what is this?)
Randi Woodbeck, Michal Harel, Joel L. Sussman, David Canner, Riley Hicks, Andrea Gorrell, Alexander Berchansky
![Figure 2. Shh-N is released from the cell membrane for long-range signaling by zinc-dependent proteolysis. [Note: This figure is adapted from references and .]](/wiki/images/thumb/4/46/Short_and_Long-Range.jpg/180px-Short_and_Long-Range.jpg)
![Figure 3. The Sonic hedgehog signaling pathway. In the absence of Shh, Patched inhibits Smo. Inhibition of Patched by Shh activates normal developmental processes. [Note: This figure is adapted from references and .]](/wiki/images/thumb/8/85/SHH_SIGNALING_PATHWAY.jpg/180px-SHH_SIGNALING_PATHWAY.jpg)
