NADPH Cytochrome P450 Oxidoreductase
From Proteopedia
(Difference between revisions)
(New page: {{STRUCTURE_3es9| PDB=3es9 | SCENE=Sandbox_177/Jmol3es9/1 }} == '''NADPH-cytochrome P450 oxidoreductase''' == ==='''General Information'''=== ---- Horecker first identified this protein i...) |
|||
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='3es9' size='350' side='right' scene='' caption='NADPH cytochrome P450 oxidoreductase complex with flavin mononucleotide, FAD and NADP, (PDB Code [[3es9]])'> | |
== '''NADPH-cytochrome P450 oxidoreductase''' == | == '''NADPH-cytochrome P450 oxidoreductase''' == | ||
| Line 12: | Line 12: | ||
==='''Structure'''=== | ==='''Structure'''=== | ||
---- | ---- | ||
| - | + | ||
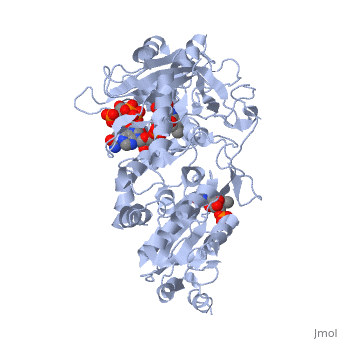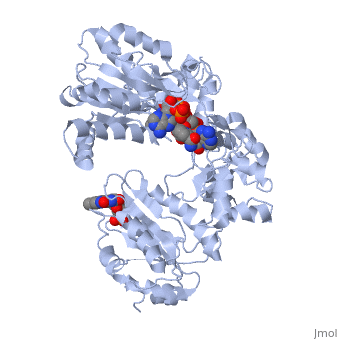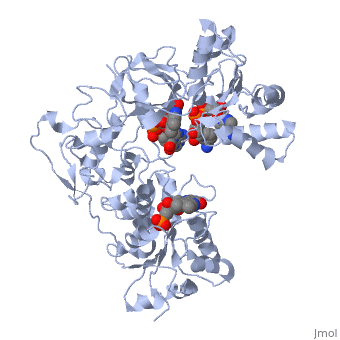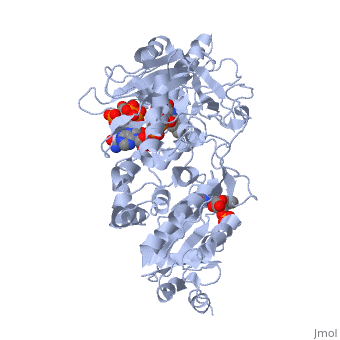
<scene name='Sandbox_177/Cyporplain/2'>CYPOR</scene> is a complex, multidomain protein composed of three asymmetric chains, (<scene name='Sandbox_177/Cyporchaina/2'>A</scene>, <scene name='Sandbox_177/Cyporchainb/1'>B</scene>, <scene name='Sandbox_177/Cyporchainc/1'>C</scene>). It also has three different types of associated ligands; one <scene name='Sandbox_177/Cyporfmn/2'>FMN</scene>, three <scene name='Sandbox_177/Cyporfad/2'>FAD</scene> and two <scene name='Sandbox_177/Cypornadph/2'>NADPH</scene> (Fig 1).<ref name="5TSON"/> The three associated binding domains for these ligand types, a connecting domain and a transmembrane anchor make up the important structural elements of CYPOR (Fig 2). | <scene name='Sandbox_177/Cyporplain/2'>CYPOR</scene> is a complex, multidomain protein composed of three asymmetric chains, (<scene name='Sandbox_177/Cyporchaina/2'>A</scene>, <scene name='Sandbox_177/Cyporchainb/1'>B</scene>, <scene name='Sandbox_177/Cyporchainc/1'>C</scene>). It also has three different types of associated ligands; one <scene name='Sandbox_177/Cyporfmn/2'>FMN</scene>, three <scene name='Sandbox_177/Cyporfad/2'>FAD</scene> and two <scene name='Sandbox_177/Cypornadph/2'>NADPH</scene> (Fig 1).<ref name="5TSON"/> The three associated binding domains for these ligand types, a connecting domain and a transmembrane anchor make up the important structural elements of CYPOR (Fig 2). | ||
| Line 22: | Line 22: | ||
---- | ---- | ||
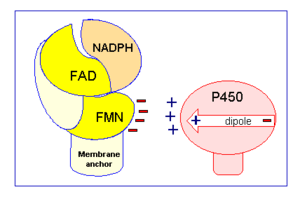
[[Image:CPR chargepair.gif|thumb|right|300px|Figure 2: Electrostatic charge pairing between Cyt P450 and the FMN binding domain induces the interaction between CYPOR and Cyt P450.]] | [[Image:CPR chargepair.gif|thumb|right|300px|Figure 2: Electrostatic charge pairing between Cyt P450 and the FMN binding domain induces the interaction between CYPOR and Cyt P450.]] | ||
| + | {{Clear}} | ||
''In vivo'' CYPOR is believe to alternate between a one and a three electron reduced form. While the 1 electron form is fairly stable, forming a neutral blue semiquinone, it is the hydroquinone, or 3 electron form, that is able to donate electrons to the desired redox partners. | ''In vivo'' CYPOR is believe to alternate between a one and a three electron reduced form. While the 1 electron form is fairly stable, forming a neutral blue semiquinone, it is the hydroquinone, or 3 electron form, that is able to donate electrons to the desired redox partners. | ||
| Line 37: | Line 38: | ||
'''Embryology & Development''' - CYPOR is believed to play a key role in the spatial and temporal expression of various signaling factors that are key in establishing correct embryogenesis and development pathways.<ref name="9TSON">PMID:11742006</ref> Studies with mice have shown that CYPOR is critical for mice embryos to progress into and past mid-gestation, as embryos lacking both CYPOR alleles did not survive past day 13.5 of gestation. <ref name="9TSON"/> Even heterozygous mice were found to have a decreased survival rate after 2 weeks of gestation.<ref name="9TSON"/> In humans, while deficiencies in CYPOR are not necessarily lethal, they do have some severe side effects, including disordered steroidogenesis and Antley-Bixler syndrome (ABS).<ref name="10aTSON"/><ref name="10bTSON">PMID:16467261</ref> ABS is associated with urogenital defects (ie: ambiguous genitalia), cranial abnormalities (ie: brachycephaly) and skeletal defects (ie: bowed femurs, narrow ribcage and club feet), often due to disordered steroidogenesis.<ref name="10aTSON"/><ref name="10bTSON"/> Individuals with ABS and/or disordered steroidogenesis may have mutations in one or both alleles for CYPOR, although some cases are associated with mutations in another gene, fibroblast growth factor receptor 2 gene.<ref name="10bTSON"/><ref name="10aTSON"/> | '''Embryology & Development''' - CYPOR is believed to play a key role in the spatial and temporal expression of various signaling factors that are key in establishing correct embryogenesis and development pathways.<ref name="9TSON">PMID:11742006</ref> Studies with mice have shown that CYPOR is critical for mice embryos to progress into and past mid-gestation, as embryos lacking both CYPOR alleles did not survive past day 13.5 of gestation. <ref name="9TSON"/> Even heterozygous mice were found to have a decreased survival rate after 2 weeks of gestation.<ref name="9TSON"/> In humans, while deficiencies in CYPOR are not necessarily lethal, they do have some severe side effects, including disordered steroidogenesis and Antley-Bixler syndrome (ABS).<ref name="10aTSON"/><ref name="10bTSON">PMID:16467261</ref> ABS is associated with urogenital defects (ie: ambiguous genitalia), cranial abnormalities (ie: brachycephaly) and skeletal defects (ie: bowed femurs, narrow ribcage and club feet), often due to disordered steroidogenesis.<ref name="10aTSON"/><ref name="10bTSON"/> Individuals with ABS and/or disordered steroidogenesis may have mutations in one or both alleles for CYPOR, although some cases are associated with mutations in another gene, fibroblast growth factor receptor 2 gene.<ref name="10bTSON"/><ref name="10aTSON"/> | ||
| + | |||
| + | </StructureSection> | ||
| + | ==3D structures of NADPH-cytochrome P450 oxidoreductase== | ||
| + | |||
| + | [[NADPH-Cytochrome P450 Reductase]] | ||
| + | |||
| + | ==Additional Resources== | ||
| + | For additional information, see: [[Cancer]] | ||
| + | <br /> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
3D structures of NADPH-cytochrome P450 oxidoreductase
NADPH-Cytochrome P450 Reductase
Additional Resources
For additional information, see: Cancer
References
- ↑ Horecker BL. Triphosphopyridine nucleotide-cytochrome c reductase in liver. J Biol Chem 1950 Apr 1;183(2):593-605
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 Hamdane D, Xia C, Im SC, Zhang H, Kim JJ, Waskell L. Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J Biol Chem. 2009 Apr 24;284(17):11374-84. Epub 2009 Jan 26. PMID:19171935 doi:10.1074/jbc.M807868200
- ↑ Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: Isolation, characterization, and kinetic studies. J Biol Chem 1962 Aug 1;237:2652-60
- ↑ 4.0 4.1 Smith GC, Tew DG, Wolf CR. Dissection of NADPH-cytochrome P450 oxidoreductase into distinct functional domains. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8710-4. PMID:8078947
- ↑ Li HC, Liu D, Waxman DJ. Transcriptional induction of hepatic NADPH: cytochrome P450 oxidoreductase by thyroid hormone. Mol Pharmacol. 2001 May;59(5):987-95. PMID:11306680
- ↑ Waxman DJ, Morrissey JJ, Leblanc GA. Hypophysectomy differentially alters P-450 protein levels and enzyme activities in rat liver: pituitary control of hepatic NADPH cytochrome P-450 reductase. Mol Pharmacol. 1989 Apr;35(4):519-25. PMID:2495435
- ↑ Ram PA, Waxman DJ. Thyroid hormone stimulation of NADPH P450 reductase expression in liver and extrahepatic tissues. Regulation by multiple mechanisms. J Biol Chem. 1992 Feb 15;267(5):3294-301. PMID:1737785
- ↑ 8.0 8.1 8.2 8.3 Tomkova M, Marohnic CC, Baxova A, Martasek P. [Antley-Bixler syndrome or POR deficiency?] Cas Lek Cesk. 2008;147(5):261-5. PMID:18630181
- ↑ 9.0 9.1 9.2 9.3 Nadler SG, Strobel HW. Identification and characterization of an NADPH-cytochrome P450 reductase derived peptide involved in binding to cytochrome P450. Arch Biochem Biophys. 1991 Nov 1;290(2):277-84. PMID:1929397
- ↑ 10.0 10.1 10.2 10.3 Tamburini PP, Schenkman JB. Differences in the mechanism of functional interaction between NADPH-cytochrome P-450 reductase and its redox partners. Mol Pharmacol. 1986 Aug;30(2):178-85. PMID:3016501
- ↑ Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J. Structure and function of cytochromes P450: a comparative analysis of three crystal structures. Structure. 1995 Jan 15;3(1):41-62. PMID:7743131
- ↑ 12.0 12.1 Canessa M, Laski C, Falkner B. Red blood cell Na+ transport as a predictor of blood pressure response to Na+ load in young blacks and whites. Hypertension. 1990 Nov;16(5):508-14. PMID:2228151
- ↑ 13.0 13.1 13.2 Shen AL, O'Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem. 2002 Feb 22;277(8):6536-41. Epub 2001 Dec 12. PMID:11742006 doi:10.1074/jbc.M111408200
- ↑ 14.0 14.1 14.2 Miller WL, Huang N, Pandey AV, Fluck CE, Agrawal V. P450 oxidoreductase deficiency: a new disorder of steroidogenesis. Ann N Y Acad Sci. 2005 Dec;1061:100-8. PMID:16467261 doi:1061/1/100
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Taya O'Neill, Joel L. Sussman, Alexander Berchansky, David Canner