Molecular Playground/ERMan1
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 4: | Line 4: | ||
| - | {{STRUCTURE_1x9d | PDB=1x9d | SCENE= }} | ||
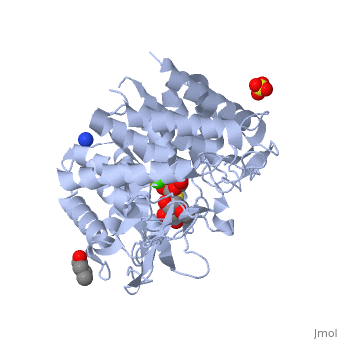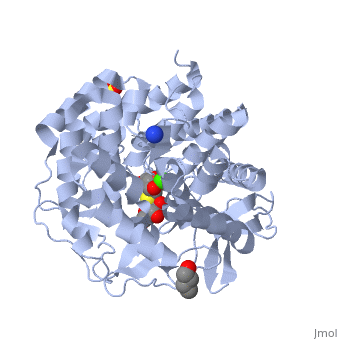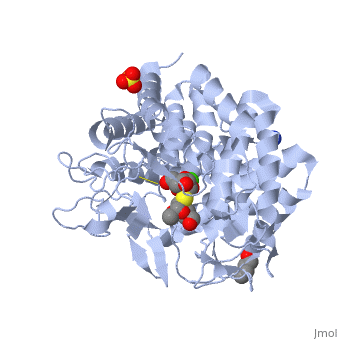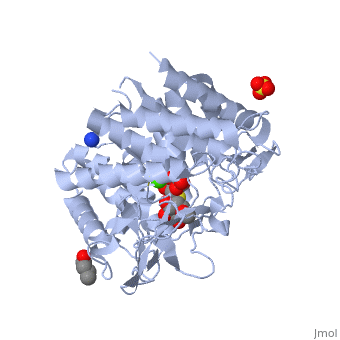
| + | {{STRUCTURE_1x9d| PDB=1x9d | SIZE=300| SCENE= |right| CAPTION=Human α-mannosidase class I complex with mannopyranoside, sulfate, 1,4-butanediol, Ca+2 ion, [[1x9d]] }} | ||
"Molecular Playground banner: A catalytic timer for protein degradation." | "Molecular Playground banner: A catalytic timer for protein degradation." | ||
| + | |||
| + | ==3D structures of mannosidases== | ||
| + | |||
| + | [[Mannosidase]] | ||
[http://proteopedia.org/wiki/index.php/CBI_Molecules] | [http://proteopedia.org/wiki/index.php/CBI_Molecules] | ||
[http://proteopedia.org/wiki/index.php/1x9d] | [http://proteopedia.org/wiki/index.php/1x9d] | ||
Current revision
ERMan1
Alpha-mannosidases I(ERMan1) is a human protein that resides in the endoplasmic reticulum(ER). ERMan1 is a member of the class 1 glycosylhydrolase family 47 group proteins and sports a Tim-barrel fold. Asparagine(N)-linked glycans are common covalent modifiers in the ER that aid in protein folding and trafficking. ERMan1 trims N-linked glycans to target a misfolded protein for degradation. The slow acting kinetics of ERMan1 have been proposed to act as a timer for protein degradation, creating a discrete window for protein folding followed by degradation if the substrate protein cannot reach the native state.
| |||||||||
| Human α-mannosidase class I complex with mannopyranoside, sulfate, 1,4-butanediol, Ca+2 ion, 1x9d | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , | ||||||||
| Gene: | MAN1B1 (Homo sapiens) | ||||||||
| Activity: | Mannosyl-oligosaccharide 1,2-alpha-mannosidase, with EC number 3.2.1.113 | ||||||||
| Related: | 1fmi | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
"Molecular Playground banner: A catalytic timer for protein degradation."