We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Atropine
From Proteopedia
(Difference between revisions)
(New page: <!-- PLEASE DO NOT DELETE THIS TEMPLATE --> {{Template:Oberholser_Sandbox_Reservation}} <!-- PLEASE ADD YOUR CONTENT BELOW HERE -->) |
|||
| (72 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
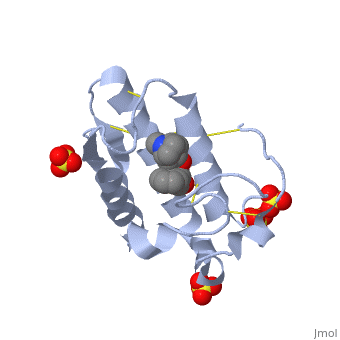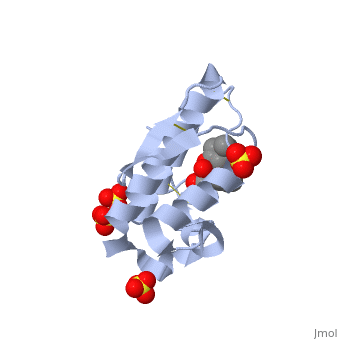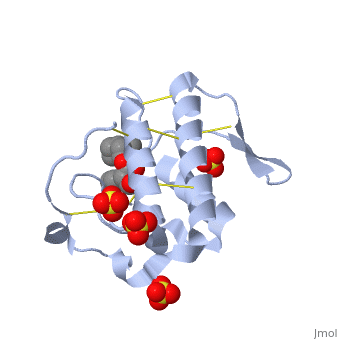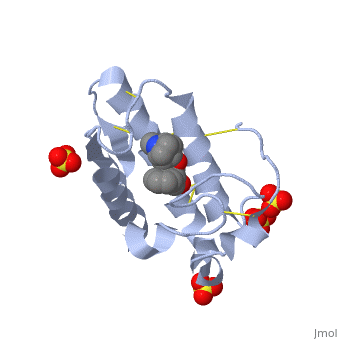
| - | < | + | <StructureSection load='1th6' size='350' side='right' scene='' caption='Phospholipase A2 complex with atropine and sulfate (PDB code [[1th6]])'> |
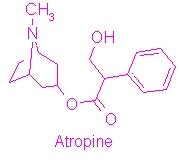
| - | {{ | + | [[Image:Atropine structure.jpg|thumb|right|1000px|Structure of Atropine]] |
| - | < | + | {{Clear}} |
| + | [[Atropine]] is an alkaloid drug derived from levohyscocyamine, a plant compound found in the family Solanaceae<ref> Atropine. Encyclopedia Brittanica. http://www.britannica.com/EBchecked/topic/42015/atropine</ref>. It's chemical name is 8-methyl-8-azabicycolo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate, and the most common medicinal form of atropine is atrophine sulfate ((C17H23NO3)2·H2SO4·H2O)<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. It is a competitive antagonist of both acetylcholine receptors and phospholipase 2A and has a variety of effects on both humans and animals. | ||
| + | |||
| + | In humans, atropine is metabolized approximately 50%, hydrolyzed to tropine and toropic acid, and the remaining unchanged drug is excreted in the urine<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. | ||
| + | |||
| + | As a plant compound, Atropine has been used for hundreds of years. Its first recorded use was in the 4th year of B.C.E, where it was used to treat wounds, gout, sleeplessnes, and was even thought to be a love potion<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. It was also used by Cleopatra and women in the Renaissance era to dilate their pupils to give them a more beautiful appearance<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. It has since been studied extensively and, although it is inherently poisonous, it is now used for a wide spectrum of medical ailments. It can be given orally, intravenously, rectally, or subcutaneously (in animals). | ||
| + | {{clear}} | ||
| + | __TOC__ | ||
| + | |||
| + | === Function and Basic Mechanism === | ||
| + | |||
| + | Atropine is part of the tropane group alkaloid family, which includes other substances such as cocaine. Atropine is a competitive antagonist of muscarinic acetylcholine receptors <ref>Gnagey, Ann L; Seidenberg, Margret; Ellis, John; ''Site-directed mutagenesis reveals two epitopes involved in the subtype selectivity of the allosteric interactions of gallamine at muscarinic acetylcholine receptors''. Molecular Pharmocology, 56:1245-1253, 1999 </ref>, a group of G-class receptor proteins, blocking the action of acetylcholine and therefore suppressing the actions of the parasympathetic nervous system<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. | ||
| + | |||
| + | ==== Acetylcholine Receptors ==== | ||
| + | |||
| + | The protein structure of an acetycholine receptor can be seen on the right. Although there is not a PDB ID for atropine in complex with the acetylcholine receptor, it is important to understand the structure of the acetylcholine receptor, and subsequently, how atropine and other alkaloids interact with it. One can see that each color in the current model represents a portion of the complex protien, which contains five domains. The <scene name='Sandbox_53/Achr_hydrophobicity/1'>hydrophobic</scene> regions can then be displayed in gray, while the hydrophillic regions appear purple. This shows a transmembrane alpha-helix region in the center of the molecule, a large hydrophillic complex on the exoplasmic face of the protein, and another hydrophillic region on the cystolic face of the protein. By highlighting the <scene name='Sandbox_53/Achr_secondarystructure/1'>secondary structure</scene> of the acetylcholine receptor, it is also easy to see how the secondary structure is concentrated to specific regions of the molecule. Alpha helicies span the majority of the transmembrane and cystolic regions, and beta sheets (with the exception of only a few alpha helicies) make up the majority of the exoplasmic receptor face. Atropine interacts with the <scene name='Sandbox_53/Achr_activesite/1'>residues</scene> of the exoplasmic face. It interacts so well, it is actually considered to be a "pure antagonist" by some <ref>Parker, Julie C; Sarkar, Deboshree; Quick, Michael W; Lester, Robin A. ''Interactions of Atropine with heterologously expressed and native alpha3 subunit-containing nicotinic acetylcholine receptors''. British Journal of Pharmacology. 138:5. p801-810. 2009. </ref>. | ||
| + | |||
| + | Atropine first enters the acetylcholine receptor and binds to the <scene name='Sandbox_53/Achr_activesite/1'>residues</scene> at the top of the receptor. They then interact with the,<scene name='Sandbox_53/Achr_residues/1'>val 255 and leu 251</scene> residues which define a hydrophobic region through which a dehydrated ion could pass through. These residues can be seen more clearly through this <scene name='Sandbox_53/Achr_residues2/1'>contacts</scene> representation. | ||
| + | |||
| + | The interaction of atropine and phospholipase 2A will be discussed in detail later in this article. | ||
| + | |||
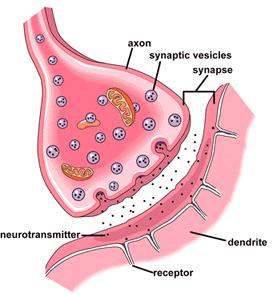
| + | [[Image:Synapse.jpg|thumb|left|350px|Synapse]]<ref>Image from: http://www.neurevolution.net/category/history/page/2/ </ref> | ||
| + | {{Clear}} | ||
| + | ==== Inhibition of Acetylcholine Receptors ==== | ||
| + | |||
| + | The image to the left depicts a synapse. A neurotransmitter, such as acetylcholine, goes across the synaptic cleft and binds to its receptor, which can be seen in great detail in the 3D image. Atropine inhibits the effect of acetylcholine by complexing the acetylcholine receptor on the other side of the cleft, subsequently inhibiting the binding of acetylcholine. If atropine does not allow acetylcholine to bind to the acetylcholine receptor, then the effects of acetylcholine are inhibited. This prevents activation of the parasympathetic nervous system and has a wide variety of medical effects. | ||
| + | |||
| + | === Medical Uses === | ||
| + | |||
| + | Since atropine affects the parasympathetic nervous system, it has a wide variety of effects. It is largely and perhaps most commonly used as an ophthalmic drug, as it paralyzes the accommodation reflex and dilates the pupil<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. The mechanism for dilation involves blocking the contraction of the circularly pupillary sphincter muscle, which is normally stimulated by acetylcholine release<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. | ||
| + | |||
| + | Outside of ophthalmic use, Atropine is also used in the treatment of heart conditions such as bradychardia (low heart rate), asystole, and subsequently, cardiac arrest. Because atropine blocks acetylcholine, and therefore the parasympathetic nervous system, the vagus nerve cannot slow the heart and it remains at a constant rate<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>. In addition, salivary, sweat, and mucus glands are inhibited by atropine, which is useful in treating asthma, or when keeping the airways of a surgical patient clear under anesthesia. Recently, it is thought to be a useful drug in patients with schizophrenia and patients suffering from neural trauma, because it reduces inflammation in the neural tissues by binding to phospholipase 2A. This will be discussed in more detail in the next section | ||
| + | |||
| + | Atropine is also a good antidote for poisoning by organophosphates and nerve gases, prime agents in bioterrorism<ref> Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine </ref>, because Atropine blocks acetylcholine at muscscarininc receptors. Atropine, however, has side effects that include nausea, dizziness, blurred visions, tachycardia, and hallucinations, and due to these side effects it has recently become more popular as a recreational drug. | ||
| + | |||
| + | [[Image:atropine.jpg|thumb|left|350px|Atropine Tablets]] <ref>Atropine Diphenoxylate. http://www.everydayhealth.com/drugs/atropine-diphenoxylate </ref> | ||
| + | {{Clear}} | ||
| + | ==== Uses in Veterinary Medicine ==== | ||
| + | |||
| + | Like human medicine, atropine is widely used in veterinary medicine in a variety of different medical situations. Although it is widely used in ophthalmic situations, it is even more commonly used as a preanesthetic in surgical patients to increase the heart rate and decrease mucous secretions, allowing the airway to remain clear during surgery. It is most often given subcutaneously, but can also be given as a pill. It is sometimes given in concjunction with morphine to decrease salivation that is often a side effect of morphine <ref> Riviere, Jim E. Papich, Mark G. Veterinary Pharmacology and Therapeutics, 9th Edition. John Wiley and Sons, 2009. </ref>. | ||
| + | |||
| + | ==== Considerations when taking Atropine ==== | ||
| + | |||
| + | First and foremost, Atropine is a toxin. Although it is now used extensively in medicine and is considered safe in the correct dosages, it is important to know the risks associated with this medication. Side effects often include dizziness, drowsiness, dry mouth, decreased sweating, dilated pupils, blurred visions, and even hallucinations <ref> ATROPINE- ORAL. http://www.medicinenet.com/atropine-oral/article.htm </ref>. Persons with allergies to alkaloids, or persons with glaucoma or asthma, and should be given only when necessary in patients with high blood pressure, liver disease, enlarged prostate, congestive heart failure, or downs syndrome<ref> ATROPINE- ORAL. http://www.medicinenet.com/atropine-oral/article.htm </ref>. | ||
| + | |||
| + | The proper dose of atropine is approximately 0.1 mg/ml in adults and 0.05 mg/ml in children when taken orally or given intravenously <ref>Atropine. http://www.rxlist.com/atropine-drug.htm </ref>. Atropine can be given orally, intravenously, rectally, or topically, and in veterinary medicine, it can be given intramuscularly or subcutaneously. | ||
| + | |||
| + | == '''Interaction of Atropine with Phospholipase A2''' == | ||
| + | |||
| + | <scene name='42/420811/Cv/1'>Atropine in complex with phospholipase A2</scene> ([[1th6]]). | ||
| + | |||
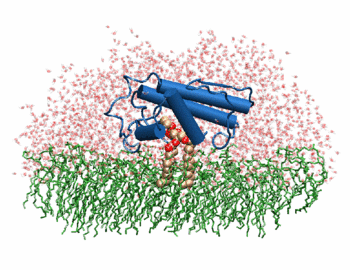
| + | [[Image:Phospholipase A2.gif|thumb|left|350px|Phospholipase 2A in complex with cell membrane]] | ||
| + | {{Clear}} | ||
| + | In addition to its ability to form complexes with acetylcholine receptors, atropine can also complex with phospholipase A2. Phospholipase A2 is a category of heat-stable enzymes which are involved in cell signaling processes, such as the inflammatory response. <ref>Kumar, Jainendra; Bala, Priti; Vihwal, Preeti. ''Analysis of Interaction of atropine with phospholipase A2 (1th6.pdb)''. Department of Botany and Biotechnlogy, College of Commerce, Patna, India.</ref>. Phospholipase 2A is an upstream regulator of inflammatory processes, and more specifically, it recognizes the sn-2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing lysophospholipids <ref> Phospholipase A2. http://www.worldlingo.com/ma/enwiki/en/Phospholipase_A2 </ref>. | ||
| + | |||
| + | This protein is found in mammals, reptile venom, and bacteria. In humans, the overproduction of phospholipase A2 leads to neurologic disorders such as schizophrenia and possibly autism <ref> Phospholipase A2. http://www.worldlingo.com/ma/enwiki/en/Phospholipase_A2 </ref>. An inhibitor of Phospholipase A2, such as Atropine, could be used to treat disorders associated with neural trauma, since Phospholipase A2 increases inflammation which could be potentially complicate neural trauma cases <ref> Phospholipase A2. http://www.worldlingo.com/ma/enwiki/en/Phospholipase_A2 </ref>. | ||
| + | |||
| + | The image to the above shows the membrane-bound phospholipase A2 in blue <ref> pla2. http://www.ks.uiuc.edu/Research/smd_imd/pla2/pla2.gif </ref>. | ||
| + | |||
| + | ==== '''Atropine in the Active Site of Phospholipase A2''' ==== | ||
| + | |||
| + | Atropine is an inhibitor of phospholipase 2A, and can be seen in complex with this enzyme on the left. The <scene name='Sandbox_53/Atropine_structure/1'>structure of atropine</scene> can be seen more clearly in gray using the ball-and stick representation of the drug and protein. It can also be seen in green in this <scene name='Sandbox_53/Phospholipase2a_composition/1'>space-filling model</scene>, where protein appears in brown, ligands appear in green, and solvents appear in blue. Finally, the | ||
| + | <scene name='Sandbox_53/Phospholipase2a_rainbow/1'>N to C terminal</scene> portions of the protein can be highlighted from blue to red in a rainbow, and the active site with atropine can be seen in the middle of the protein. | ||
| + | |||
| + | Atropine interacts with phospholipase 2A at residues asp29 and tyr49 on the protein. The | ||
| + | <scene name='Sandbox_53/Phospholipase2a_residues/1'>residues</scene> of atropine interacting with phospholipase 2A can be seen on the right. The amino acid residues in the active site are labeled. As seen in the acetylcholine receptor, the <scene name='Sandbox_53/Phospholipase_hyrophobic/1'>hydrophobic</scene> regions of the phospholipase 2A enzyme are found in the active site, which is where the atropine binds and inhibits the enzyme. The hydrophobic regions, represented in gray, can be seen surrounding atropine, which is positioned in the active site and capped by red oxygen atoms. | ||
| + | |||
| + | Removing the labels, atropine can be seen making contact with the atoms emphasized by the space filling model, interacting with the <scene name='Sandbox_53/Phospholipase2a_interactions/1'>active site</scene> of phospholipase 2A through white as-tricks. | ||
| + | </StructureSection> | ||
| + | == '''References''' == | ||
| + | <references/> | ||
Current revision
| |||||||||||
References
- ↑ Atropine. Encyclopedia Brittanica. http://www.britannica.com/EBchecked/topic/42015/atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Gnagey, Ann L; Seidenberg, Margret; Ellis, John; Site-directed mutagenesis reveals two epitopes involved in the subtype selectivity of the allosteric interactions of gallamine at muscarinic acetylcholine receptors. Molecular Pharmocology, 56:1245-1253, 1999
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Parker, Julie C; Sarkar, Deboshree; Quick, Michael W; Lester, Robin A. Interactions of Atropine with heterologously expressed and native alpha3 subunit-containing nicotinic acetylcholine receptors. British Journal of Pharmacology. 138:5. p801-810. 2009.
- ↑ Image from: http://www.neurevolution.net/category/history/page/2/
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine. New World Encyclopedia. http://www.newworldencyclopedia.org/entry/Atropine
- ↑ Atropine Diphenoxylate. http://www.everydayhealth.com/drugs/atropine-diphenoxylate
- ↑ Riviere, Jim E. Papich, Mark G. Veterinary Pharmacology and Therapeutics, 9th Edition. John Wiley and Sons, 2009.
- ↑ ATROPINE- ORAL. http://www.medicinenet.com/atropine-oral/article.htm
- ↑ ATROPINE- ORAL. http://www.medicinenet.com/atropine-oral/article.htm
- ↑ Atropine. http://www.rxlist.com/atropine-drug.htm
- ↑ Kumar, Jainendra; Bala, Priti; Vihwal, Preeti. Analysis of Interaction of atropine with phospholipase A2 (1th6.pdb). Department of Botany and Biotechnlogy, College of Commerce, Patna, India.
- ↑ Phospholipase A2. http://www.worldlingo.com/ma/enwiki/en/Phospholipase_A2
- ↑ Phospholipase A2. http://www.worldlingo.com/ma/enwiki/en/Phospholipase_A2
- ↑ Phospholipase A2. http://www.worldlingo.com/ma/enwiki/en/Phospholipase_A2
- ↑ pla2.
Proteopedia Page Contributors and Editors (what is this?)
Lindsey Hayes, David Canner, Alexander Berchansky, Michal Harel, OCA