We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Collagen Structure & Function
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1cag' size='450' side='right' scene='Sandbox_168/Default/3' caption=''> | |
==Introduction== | ==Introduction== | ||
| - | Collagen is a member of a family of naturally occurring proteins. It is one of the most plentiful proteins present in mammals and it is responsible for performing a variety of important biological functions. It is most well-known for the structural role it plays in the body. It is present in large quantities in connective tissue and provides tendons and ligaments with tensile strength and skin with elasticity. It often works in conjuction with other important proteins such as keratin and elastin. | + | [[Collagen]] is a member of a family of naturally occurring proteins. It is one of the most plentiful proteins present in mammals and it is responsible for performing a variety of important biological functions. It is most well-known for the structural role it plays in the body. It is present in large quantities in connective tissue and provides tendons and ligaments with tensile strength and skin with elasticity. It often works in conjuction with other important proteins such as keratin and elastin. |
==Biosynthesis== | ==Biosynthesis== | ||
| Line 14: | Line 14: | ||
These three α-chains are then twisted around one another in a rope-like manner to produce the overall tightly packed triple-helical form of the molecule. The interaction of α-chains is stabilized via interchain hydrogen bonding making the molecule fairly resistant to attack by other molcules. Each α-chain is surrounded by a hydration sphere which allows a hydrogen bonding network to be present between the water molecules and the peptide acceptor groups.<ref name="collalike" />. This hydrogen bonding occurs when the amino group (NH) of a glycine residue forms a peptide bond with the carbonyl (C=0) of an adjacent residue. The overall molecule is approxiametly 300nm long and 1.5-2nm in diameter.<ref name="collalike" />. | These three α-chains are then twisted around one another in a rope-like manner to produce the overall tightly packed triple-helical form of the molecule. The interaction of α-chains is stabilized via interchain hydrogen bonding making the molecule fairly resistant to attack by other molcules. Each α-chain is surrounded by a hydration sphere which allows a hydrogen bonding network to be present between the water molecules and the peptide acceptor groups.<ref name="collalike" />. This hydrogen bonding occurs when the amino group (NH) of a glycine residue forms a peptide bond with the carbonyl (C=0) of an adjacent residue. The overall molecule is approxiametly 300nm long and 1.5-2nm in diameter.<ref name="collalike" />. | ||
| - | The image on the right-hand side has each side chain colored a different color to shown how each individual <scene name='Sandbox_168/Helices/1'> | + | The image on the right-hand side has each side chain colored a different color to shown how each individual <scene name='Sandbox_168/Helices/1'>helices</scene> interact with the others to form the overall molecule. The <scene name='Sandbox_168/Myscene/1'>active sites</scene> |
have also been illustrated to point out their positions in the triple-helix. | have also been illustrated to point out their positions in the triple-helix. | ||
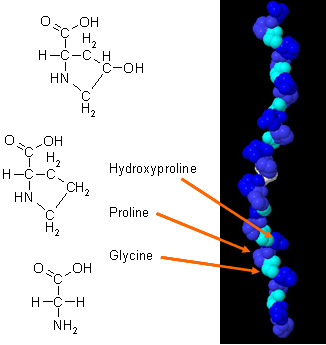
| - | [[Image:collagen_(alpha_chain).jpg | thumb |'''Figure 1.''' Amino Acid residues in collagen. Gly, Pro and Hydroxyproline residues present in a collagen molecule <ref name="residues"/>.]] | + | [[Image:collagen_(alpha_chain).jpg |400px| thumb |'''Figure 1.''' Amino Acid residues in collagen. Gly, Pro and Hydroxyproline residues present in a collagen molecule <ref name="residues"/>.]] |
| - | + | {{Clear}} | |
==Function== | ==Function== | ||
There are close to 30 different types of collagen that have been identified so far.<ref name="types">PMID:17581806</ref>. | There are close to 30 different types of collagen that have been identified so far.<ref name="types">PMID:17581806</ref>. | ||
| Line 41: | Line 41: | ||
*Atopic Dermatitis (III) | *Atopic Dermatitis (III) | ||
| - | + | </StructureSection> | |
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 PMID:PMC1367617
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Bella J, Eaton M, Brodsky B, Berman HM. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science. 1994 Oct 7;266(5182):75-81. PMID:7695699
- ↑ 3.0 3.1 Yamazaki CM, Kadoya Y, Hozumi K, Okano-Kosugi H, Asada S, Kitagawa K, Nomizu M, Koide T. A collagen-mimetic triple helical supramolecule that evokes integrin-dependent cell responses. Biomaterials. 2010 Mar;31(7):1925-34. Epub 2009 Oct 22. PMID:19853297 doi:10.1016/j.biomaterials.2009.10.014
- ↑ Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929-58. PMID:19344236 doi:10.1146/annurev.biochem.77.032207.120833
- ↑ 5.0 5.1 Koide T. Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering. Philos Trans R Soc Lond B Biol Sci. 2007 Aug 29;362(1484):1281-91. PMID:17581806 doi:10.1098/rstb.2007.2115
Proteopedia Page Contributors and Editors (what is this?)
Daman K. Kandola, Alexander Berchansky, David Canner, Andrea Gorrell, Luis Netto