Introduction
Theodor Schwann discovered Pepsin in 1836. He named the enzyme pepsis from the Greek word peptein which means to digest. Pepsin was the first animal enzyme to be discovered, and, in 1929, it became one of the first enzymes to be crystallized, by John H. Northrop.
Pepsin was also the first crystallized protein to be studied by X-ray diffraction using the method of capillary mounting to prevent water loss [1].
- Pepsinogen is the inactive precursor of pepsin. It is converted to the active pepsin by the hydrochloric acid in the stomach[2].
- Endothiapepsin is pepsin from Endothia parasitica which is used for fragment screening[3].
See also:
Protein Function
Pepsin is one of three proteolytic, or protein degrading enzymes in the digestive system. It resides in the alimentary canal, and is produced by mucosal cells, as an inactive precursor pepsinogen. Once it is secreted into the acidic conditions of the stomachs lumen, the propart peptide is cleaved, yielding the active pepsin. Pepsin degrades peptides, and is optimally active at low pHs [1]. Pepsin is an aspartic proteinase, more specifically a eukaryotic aspartic protease enzyme. Pepsin was among the first enzymes to be isolated in crystalline form [4]. Aspartic proteinases are widespread in nature, and pepsin in particular has been known to be medically important [5]. Endothiapepsin (ETPep) hydrolyzes proteins with preference to hydrophobic residues at P1 and P1' positions.
Overall Structure
Pepsin is bilobal, and composed of two nearly equal N and C domains related by an intra dyad [4]. There are 326 residues in pepsin, forming two topologically similar lobes. Residues 1-175 form the N-terminal lobe, and residues 176-327 constitute the C-terminal lobe. A large portion of the residues are polar and buried [1]. Each of these domains consists predominantly of β-sheets [4]. In fact, 44% of the structures residues are within a β-sheet. There are six small right-handed α-helical segments, the longest being hc which spans from residues 225-236. All the a-helices except h'c are partially exposed and have some amphiphilic characteristics especially hc. Hc has a solvated surface and a buried side [1]. The two most prominent strands of mixed β-sheets in both domains are 1N and 1C sheets. These sheets are related by an intra-lobe topological 2-fold symmetry. The most important β-sheet consists of six anti-parallel β-strands. “Two further β-sheets, 2N and 2C are each related by an intra-lobe topologically related β-hairpins, folded below the 1N and 1C sheets. Further, a six-stranded sheet spans the two lobes and forms a structure resembling an arch upon which the other four strands reside [1].” The overall peptide folds and active site structures are homologous [4]. Aspartic proteinases, including pepsin are distinguishable by the presence of two conserved aspartic acid residues in the active site [4]. Each domain in pepsin contains one of two catalytically important aspartic acid residues [4]. The interface between sheets 1N and 1C forms the catalytic center consisting of the two nearly co-planar carboxyl groups of aspartate residues , that are held in close proximity by a network of hydrogen bonds, and are shielded from solvents by a β-hairpin loop. Both catalytic residues can be protonated, although only one carboxyl group can be negatively charged at any one time [1]. The side chains are involved in hydrogen-bond interactions with the main chain of the protein or other conserved side-chains of the enzyme [1].” Hydrogen-bonds are what stabilize the fold of pepsin. This stabilization is called the fireman`s grip. About 188 main-chain-main –chain hydrogen bonds exist. The enzyme also has a high proportion of serine and threonine residues, the total being 60. Of these, 32 are involved with side-chain hydrogen-bond interactions with other residues of the enzyme. There are 2425 non-hydrogen protein atoms and 371 water molecules [1] . Pepsin has a very low pI in the range of 2-3 pH units, which is due to the high proportion of carboxyl residues, there is a total of 43. The protein is phosphorylated at Ser68 and has three disulphide bridges. Furthermore, pepsin contains several salt-bridges. One salt-bridge connecting the molecules at the 206 to 210 positions enclose the pepsins only type II turn. Type I turns are the most prevalent in pepsin [1]. Lastly, are typically three basic residues in pepsin, Arg308, His53, and Lys320.
What are the Structures Implications?
One of the main salt bridges in pepsin includes residues from one of the dyad-related hylices (h’N and h’C) and a residue from the opposite lobe, e.g. Asp138 interacts with Arg316. For an image of these residues refer to image 1. bellow. This ion-pair may be important for the stability of the pepsin fold, since chemical modification of Arg316 with butane-2,3-dione partially inactivates the enzyme, as does treatment with phenylglyoxal [1].
Image 1. The hydrogen bonds between Asp138 and Arg316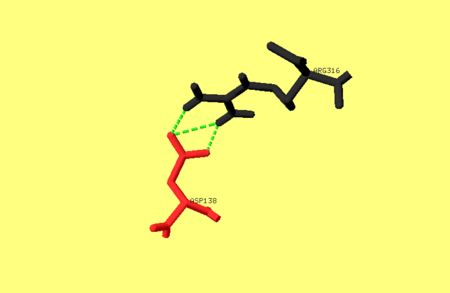
There are many sub domains in pepsin. One sub domain which includes the residues 222-235; 255-276; and 283-290. Portions of this sub domains can interact with inhibitors, and therefore contribute to the structural delineation of S’ sub sites [4]. It is also possible that ridged movements of sub domains relative to the large domain may help the enzyme adjust to various substrate structures. Furthermore, local regional flexibility of structures such as the “flap,” in and around the active site of pepsin has been suggested to modulate substrate and inhibitor binding [4].
Once denatured, pepsin is unable to refold to an active native state upon returning from denaturing conditions. One proposed solution for this is that pepsin formation depends on a separate prosegment (PS) domain. When returning from the denatured state, the denatured pepsin first has to bypass a large folding barrier and then in the presence of PS the native state can become thermodynamically stable. The PS therefore can catalyze pepsin folding by stabilizing the transition state [5] .

