Phosphoinositide 3-Kinases
From Proteopedia
(Difference between revisions)
| (52 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | {{BAMBED | ||
| + | |DATE=November 15, 2010 | ||
| + | |OLDID=1144475 | ||
| + | |BAMBEDDOI=10.1002/bmb.20540 | ||
| + | }} | ||
| + | <StructureSection load='3hhm' size='350' side='right' scene='' caption='PI3K (grey) complex with NISH2 P85α and wortmannin (PDB code [[3hhm]]) '> | ||
| + | |||
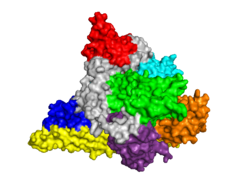
[[Image: PI3KOpener.PNG|250px|left|thumb| PI3K p110α Subunit, [[3hhm]]]] | [[Image: PI3KOpener.PNG|250px|left|thumb| PI3K p110α Subunit, [[3hhm]]]] | ||
| - | {{ | + | {{Clear}} |
| - | [[Phosphoinositide 3-Kinases]] (PI3K) are a family of ubiquitously distributed lipid kinases, that play a critical role in the regulation of numerous cellular processes including cellular growth and morphology, programmed cell death, cell motility and adhesion, mitogenesis and glucose uptake. <ref name="Driscoll"> PMID: 12151228</ref> PI3K generates important second messengers by catalyzing the transfer of the γ-phosphate group of ATP to the D3 position of phosphoinositides. <ref name="Wymann"> PMID: 9838078</ref> The PI3K preferred substrate is Phosphatidylinositol-4,5-bisphosphate (PIP2), which is converted into phosphatidylinositol-3,4,5-triphosphate (PIP3) upon phosphorylation at the cell membrane. The importance of PI3K is evident in knockout mice studies in which those mice with disruptions of critical PI3K components have significant deficiencies in immune and inflammatory response <ref name="Fubar"> PMID:10972292</ref> sometimes resulting in embryonic death.<ref>PMID:10196176</ref> Aberrations in PIP3 levels, either through activation of PI3ks or through inactivation of lipid phosphatase [[PTEN]], occur frequently in numerous forms of cancer, making PI3K an exciting new target to treat [[Cancer|cancer]] among other human diseases.<ref name="Miled"> PMID: 17626883</ref> | + | __TOC__ |
| - | + | == Function == | |
| + | [[Phosphoinositide 3-Kinases]] or '''phosphatidylinositol 3-kinase''' (PI3K) are a family of ubiquitously distributed lipid kinases, that play a critical role in the regulation of numerous cellular processes including cellular growth and morphology, programmed cell death, cell motility and adhesion, mitogenesis and glucose uptake. <ref name="Driscoll"> PMID: 12151228</ref> PI3K generates important second messengers by catalyzing the transfer of the γ-phosphate group of ATP to the D3 position of phosphoinositides. <ref name="Wymann"> PMID: 9838078</ref> The PI3K preferred substrate is Phosphatidylinositol-4,5-bisphosphate (PIP2), which is converted into phosphatidylinositol-3,4,5-triphosphate (PIP3) upon phosphorylation at the cell membrane. The importance of PI3K is evident in knockout mice studies in which those mice with disruptions of critical PI3K components have significant deficiencies in immune and inflammatory response <ref name="Fubar"> PMID:10972292</ref> sometimes resulting in embryonic death.<ref>PMID:10196176</ref> Aberrations in PIP3 levels, either through activation of PI3ks or through inactivation of lipid phosphatase [[PTEN]], occur frequently in numerous forms of cancer, making PI3K an exciting new target to treat [[Cancer|cancer]] among other human diseases.<ref name="Miled"> PMID: 17626883</ref> For additional details see<br /> | ||
| + | * [[PI3K Activation, Inhibition, & Medical Implications]]<br /> | ||
| + | * [[Human PI3K p110alpha/p85alpha]]<br /> | ||
| + | * [[The Structure of PI3K]]<br /> | ||
| + | * [[Akt/PKB signaling pathway]]<br /> | ||
| + | * [[Diabetes & Hypoglycemia]]. | ||
| + | |||
==The Classes of PI3Ks== | ==The Classes of PI3Ks== | ||
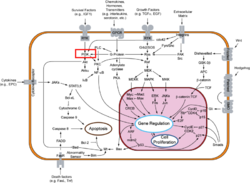
| - | [[Image:PI3KTransduction.PNG|250px|left|thumb| Signal Transduction Pathway. PI3K Highlighted in Red. Click to Expand]] PI3Ks can be grouped into three distinct classes, Class I-III. Class I PI3Ks, the most well understood and thoroughly explored PI3K class, are composed of a 110kDa <scene name='Phosphoinositide_3-Kinases/Model_cat/2'>catalytic subunit</scene> and a 50-100 kDa <scene name='Phosphoinositide_3-Kinases/Model_ada/1'>adaptor subunit</scene>. Activation of Class I PI3Ks is controlled by extracellular signaling via receptors with intrinsic tyrosine kinase activity, G protein-linked receptors, or receptors coupled to [[SRC]] like protein tyrosine kinases. <ref>PMID:1851250</ref> Class II PI3Ks are relatively poorly understood but are 170-210 kDa and have in vitro substrate specificity toward PtdIns 4-P. Class III PI3Ks depend on Vps15p protein Ser/Thr kinases, which recruits the phosphatidylinositol kinase to late Golgi Compartments. <ref name="Wymann"/> | + | [[Image:PI3KTransduction.PNG|250px|left|thumb| Signal Transduction Pathway. PI3K Highlighted in Red. Click to Expand]] |
| + | {{Clear}} | ||
| + | PI3Ks can be grouped into three distinct classes, Class I-III. | ||
| + | *'''Class I PI3Ks''', the most well understood and thoroughly explored PI3K class, are composed of a 110kDa <scene name='Phosphoinositide_3-Kinases/Model_cat/2'>catalytic subunit</scene> and a 50-100 kDa <scene name='Phosphoinositide_3-Kinases/Model_ada/1'>adaptor subunit</scene>. Activation of Class I PI3Ks is controlled by extracellular signaling via receptors with intrinsic tyrosine kinase activity, G protein-linked receptors, or receptors coupled to [[SRC]] like protein tyrosine kinases. <ref>PMID:1851250</ref> | ||
| + | *'''Class II PI3Ks''' are relatively poorly understood but are 170-210 kDa and have in vitro substrate specificity toward PtdIns 4-P. | ||
| + | *'''Class III PI3Ks''' depend on Vps15p protein Ser/Thr kinases, which recruits the phosphatidylinositol kinase to late Golgi Compartments. <ref name="Wymann"/> | ||
===Class I Subclasses=== | ===Class I Subclasses=== | ||
| - | PI3Ks are activated by extracellular agonists via the translocation of PI3Ks to the plasma membrane for easy access to lipid substrates. Depending on the adaptor proteins involved in the process, Class I PI3Ks are segregated into two subgroups. Those that associate with p85 will be directed to phosphorylated tyrosine motifs (Class IA), | + | PI3Ks are activated by extracellular agonists via the translocation of PI3Ks to the plasma membrane for easy access to lipid substrates. Depending on the adaptor proteins involved in the process, Class I PI3Ks are segregated into two subgroups. Those that associate with p85 will be directed to phosphorylated tyrosine motifs (Class IA), '''Phosphatidylinositol-4, 5-bisphosphate 3-kinase''' (PI3Kγ) catalyzes the conversion of 1-phosphatidyl-1D-myo-inositol-4, 5-bisphosphate and ATP to 1-phosphatidyl-1D-myo-inositol-4, 5-trisphosphate. PI3Kγ interacts with trimeric G proteins and the p101 protein (Class IB) <ref name="Wymann"/> |
==Structure of PI3K== | ==Structure of PI3K== | ||
| - | + | For Full Article, See: [[The Structure of PI3K]] <br /> | |
| - | <br /> | + | |
| - | + | Class I PI3Ks, which are tightly regulated by tyrosine kinases, are composed of an 85kDa regulatory/adapter subunit (p85) and a 110kDa catalytic subunit (p110). <ref name="Flip"> PMID: 10525402</ref> | |
| - | + | ||
| - | + | ||
| - | Class | + | |
| - | + | ||
| - | + | ||
| - | + | ||
<br /> | <br /> | ||
| - | ====Proline-Rich Regions==== | ||
| - | The proline rich regions which flank the BH domain are ideal ligands for various SH3 containing non-receptor protein tyrosine kinases like [[Src]], Lyn & Fyn, often with the product of the proteo-oncogene product Cbl as a docking site. <ref>PMID:9160881</ref> | ||
| - | <br /> | ||
| - | == | + | ==PI3K Activation, Inhibition, and Medical Implications== |
| - | + | For Full Article, See: [[PI3K Activation, Inhibition, & Medical Implications]] <br /> | |
| - | <br /> | + | |
| - | + | A number of inhibitors for PI3K have been developed to understand how PI3K is activated and functions. These analysis have massive medical implications for the treatment of [[Cancer]] and [[Diabetes]]. Inhibitors of Type I PI3K p110γ and Type I PI3K p110δ are tested as therapeutic drugs against inflammatory etiologists <ref>PMID:19876783</ref>. | |
| - | + | <br/> | |
| - | <br /> | + | |
| - | == | + | == 3D Structures of PI3K== |
| - | + | [[Phosphoinositide 3-kinase 3D structures]] | |
| - | + | ||
| - | ==Regulation of Class IA PI3K via p85 Phosphorylation== | ||
| - | All PI3K catalytic subunits possess intrinsic protein serine kinase activity. PI3K regulatory subunits can be phophorylated by the catalytic subunit (p110) at specific sites. For example, phophorylation of Ser 608, a residue located in an area of the iSH2 domain that is critical for PIP2 presentation to the catalytic subunit, results in a dramatic reduction in PI3K lipid kinase activity.<ref>PMID: 8313897</ref> Additionally, tyrosines 580 and 607 can be phosphorylated upon stimulation with insulin and growth factor along with <scene name='User:David_Canner/Sandbox_P/Tyr_508/1'>Tyr 508 upon PDGF receptor mediation</scene>. <ref name="Wymann"/> Phosphorylation of Tyr 688 in the CSH2 domain by Abl and Lck results in reduced affinity for phosphopeptides and subsequent activation of the catalytic domain. <ref>PMID:9461588 </ref> | ||
| - | <br /> | ||
| - | __NOTOC__</StructureSection> | ||
| - | |||
| - | ==The Catalytic Subunit== | ||
| - | <StructureSection load='1dq8' size='500' side='right' scene='User:David_Canner/Sandbox_P/Full/4' caption='Structure of PI3K p110, ([[3hhm]])'> | ||
| - | ===The Catalytic Subunit (P110) of Class 1 PI3Ks=== | ||
| - | The catalytic subunit, P110 has several isoforms that associate with different classes of PI3Ks. P110α, β, and δ associate with Class IA PI3Ks while p110γ associates with Class 1B PI3ks. <ref name="Wymann"/> The <scene name='User:David_Canner/Sandbox_P/Full/1'>p110α subunit contains several domains including</scene> an N-terminal adaptor-binding domain (ABD), a Ras binding domain (RBD) a C2 domain that likely binds to the cellular membrane, a helical domain (HD) with unknown function, and the actual catalytic kinase domain. <ref name="Amzel"> PMID: 19805105 </ref> The actions of these domains are coordinated by the nSH2 communicating domain in p85. | ||
| - | <br /> | ||
| - | |||
| - | ===Communication between nSH2 & The Catalytic Subunit Domains=== | ||
| - | <scene name='User:David_Canner/Sandbox_P/Nsh2_full/1'>The alpha-A helix of NSH2 </scene> (residues 340-345) is anchored into <scene name='User:David_Canner/Sandbox_P/Nsh2_pocket/2'> a cavity created by the C2 and Kinase domain interface.</scene> Helix α11K of the <scene name='User:David_Canner/Sandbox_P/Kinase_domain_out/2'>Kinase domain</scene> (residues 1017-1024) <scene name='User:David_Canner/Sandbox_P/Nsh2_kianse/1'>interacts with the alpha-A helix of nSH2.</scene> nSH2 interacts with the <scene name='User:David_Canner/Sandbox_P/C2_out/3'>C2 domain</scene> through a network of charge-charge interactions involving two loops on nSH2 (Residues 374-377 & 350-354) and C2 residues 364-371, a strong <scene name='User:David_Canner/Sandbox_P/Nsh2_charge_charge/3'>salt bridge between NSH2 Glu 349 and C2 residue Arg 357, and hydrogen bonds between NSH2 Glu 348 and C2 Glu 453 and Asp 454.</scene> <ref name="Amzel"/> | ||
| - | <br /> | ||
| - | |||
| - | The <scene name='User:David_Canner/Sandbox_P/Helical_overview/2'>helical domain in p110</scene>, whose function isn’t thoroughly understood, interacts with nSH2 via charge interactions. The HD residue, <scene name='User:David_Canner/Sandbox_P/Helical_domain/1'>Glu 542 forms a slat bridge with Arg 358 on NSH2 while Glu 545 interacts with NSH2 Lys 379</scene>. These residues are known hotspot mutations which are associated with various types of cancer. <ref name="Amzel"/> This loop in <scene name='User:David_Canner/Sandbox_P/Nsh2__and_helical_ligand_out/2'>the helical domain </scene> which contains the hotspots (residues 542-546) is located precisely where <scene name='User:David_Canner/Sandbox_P/Nsh2_ligand_just_ligand_full/1'> the phosphopeptide of NSH2 ligands, like PDGFR, bind to NSH2.</scene> The salt bridge formed between <scene name='User:David_Canner/Sandbox_P/Nsh2_disruption_of_salt/1'>Glu 542 and nSH2 is disrupted upon binding phosphorylated peptides</scene> like PDGFR, eliminating nSH2-mediated inhibition of p110α and activating the enzyme to phosphorylate PIP2 into PIP3. The hotspot mutation at Glu 542 accomplishes the same thing by eliminating the salt bridge and uninhibiting p110α. It is the <scene name='User:David_Canner/Sandbox_P/Kinase_with_atp_full/2'>kinase domain </scene> which <scene name='User:David_Canner/Sandbox_P/Kinase_with_atp_zoomed/3'>binds ATP to provide the phosphate group</scene> used to convert PIP2 into PIP3. <ref name="Amzel"/> | ||
| - | |||
| - | ===Model for Catalysis=== | ||
| - | Although no <scene name='User:David_Canner/Sandbox_P/Inhibitor_main/4'>crystal structure of PI3K</scene> with bound substate analog has been solved, a model for PIP2 phosphorylation has been developed and is generally supported. <ref name="Walker2">PMID:10580505</ref> In this model, the headgroup of PIP2 is <scene name='User:David_Canner/Sandbox_P/Catalytic_cavity/2'>positioned in a cavity</scene> between the <scene name='User:David_Canner/Sandbox_P/Catalytic_site/1'>C-terminal helix 12 of the kinase domain, the “activation” loop, and the “catalytic” loop</scene>. This puts the 5-phosphate of PIP2 near Lys 973 and the <scene name='User:David_Canner/Sandbox_P/Catalytic_site_atp_lys/1'>I-phosphate of ATP near Lys 807 and Lys 808</scene>. The <scene name='User:David_Canner/Sandbox_P/Catalytic_site_pip2/1'>basic residues Arg 947</scene> and Lys 973 can bind the 4-Phosphate of PIP2 and help provide the Class I PI3Ks with their specificity for PIP2. Once PIP2 and ATP are bound, it is believed <scene name='User:David_Canner/Sandbox_P/Catalytic_site_his/1'>His 948 rotates to interact with PIP2</scene>, deprotonating it at the C-3 Hydroxyl position creating a nucleophile. This nucleophile subsequently attacks the gamma phosphate of ATP producing PIP3. <ref name="Walker2"/> | ||
</StructureSection> | </StructureSection> | ||
| - | |||
| - | ==Activation of Class IA PI3K== | ||
| - | Inactive PI3Ks are rapidly activated in the presence of extracellular stimuli. Such stimuli, as discussed previously, include growth factor receptors with intrinsic protein tyrosine kinase activity, which display pYXXM motifs for p85 docking, as well as receptor substrates which are phosphorylated and interact with PI3K regulatory subunits like nSH2. PI3K can be additionally activated in cooperative processes like translocation to the plasma membrane where lipid substrates are available and by binding GTP loaded Ras to the catalytic subunit. <ref>PMID: 8402898</ref>. <ref name="Wymann"/> | ||
| - | <br /> | ||
| - | |||
| - | ==PI3K Inhibition== | ||
| - | <StructureSection load='1dq8' size='500' side='left' scene='User:David_Canner/Sandbox_P/Full/4' caption='Structure of PI3K p110, ([[3hhm]])'> | ||
| - | ===Inhibition by Wortmannin, LY294002 & Others: Implications=== | ||
| - | Wortmannin is an irreversible inhibitor of PI3-Kinases by alkylating a lysine residue at the putative ATP binding site of p110. <ref>PMID:9838078</ref> LY294002 is a competitive inhibitor of ATP. <ref name="Stein"> PMID:11566615</ref> Due to their instability and lack of selectivity leading to toxicity, neither wortmannin nor LY294002 are not valid pharmaceutical therapeutics. <ref name="Stein"/> That being said, these compounds along with Quercetin, Myricetin and Staurosporine, can serve as excellent tools for investigating PI3K structure. Further, derivatives of wortmannin with more favorable pharmacological profiles are currently in clinical trials for various PI3K associated diseases. <ref>PMID: 1474947</ref> | ||
| - | <br /> | ||
| - | |||
| - | ===Wortmannin Binding=== | ||
| - | <scene name='User:David_Canner/Sandbox_P/Wortmannin/2'>Wortmannin</scene> binds the <scene name='User:David_Canner/Sandbox_P/Kinase_domain_out/4'>p110 kinase domain</scene> ATP-binding site, <scene name='User:David_Canner/Sandbox_P/Kinase_bound_wortmannin/2'>positioning itself in a conserved pocket</scene> using conserved p110α residues <scene name='User:David_Canner/Sandbox_P/Wortmannnin_binding/1'>Ile 800, Ile 848, Val 850, Val 851, Ser 919, Met 922, Phe 930, Ile 932 and Asp 933</scene>. Wortmannin forms <scene name='User:David_Canner/Sandbox_P/Wortmannnin_binding_for_reals/1'>a covalent bond with Lys 802 and hydrogen bonds with Asp 933, Tyr 836, Val 851 and Gln 859</scene> Inhibition of the ATP binding site prevents binding of ATP and subsequent transfer of the γ-phosphate group of ATP to PIP2. <ref name="Amzel"/> | ||
| - | <br /> | ||
| - | |||
| - | ===LY294002, Quercetin, Myricetin & Staurosporine=== | ||
| - | LY294002, a competitive inhibitor of ATP binding in the PI3K kinase domain, was first discovered by scientists at Eli Lilly. Quercetin, Myricetin & Staurosporine are natural compounds which broadly inhibit protein kinases. <ref name="Walker"> PMID:11090628</ref> Understanding how ATP binds to the ATP binding site <scene name='User:David_Canner/Sandbox_P/Inhibitor_main/4'>within the kinase domain</scene> of PI3Kγ and how various inhibitors prevent this interaction helps elucidate ways to develop effective, selective inhibitors. See p110γ bound to <scene name='User:David_Canner/Sandbox_P/Inhibitor_atp/5'>ATP</scene> ([[1e8x]]), <scene name='User:David_Canner/Sandbox_P/Inhibitor_wortmannin/7'>Wortmannin</scene> ([[1e7u]]), <scene name='User:David_Canner/Sandbox_P/Inhibitor_ly294002/2'>LY294002</scene> ([[1e7v]]), <scene name='User:David_Canner/Sandbox_P/Inhibitor_quer/2'>Quercetin</scene> ([[1e8w]]), <scene name='User:David_Canner/Sandbox_P/Inhibitor_staur/1'>Staurosporine</scene> ([[1e8z]]), <scene name='User:David_Canner/Sandbox_P/Inhibitor_myrice/1'>Myricetin</scene> ([[1e90]]).<ref name="Walker"/> | ||
| - | <br /> | ||
| - | </StructureSection> | ||
| - | |||
| - | ==The Phosphorylated Lipid Products in Downstream Signaling== | ||
| - | Ligand receptor interactions trigger a rapid rise of cellular PIP3. Numerous molecular targets are activated upon interaction with PIP3. One such target is the Ser/Thr kinase Akt, which requires the action of phosphoinositide dependent kinases, another step for potential fine tuning. Akt subsequently inactivates glycogen-synthase-kinase 3 and the pro-apoptotic factor BAD. <ref>PMID:9346240</ref>. PIP3 also activates Btk, an essential protein for normal B lymphocyte development and function <ref>PMID: 8162018</ref> along with dozens of other targets including centaurin, profiling, cytohesin, etc. which control<ref name="Wymann"/> | ||
| - | <br /> | ||
| - | |||
| - | ==Medical Implications== | ||
| - | <StructureSection load='1dq8' size='500' side='right' scene='User:David_Canner/Sandbox_P/Full/4' caption='Structure of PI3K p110, ([[3hhm]])'> | ||
| - | ===PI3K In Medicine=== | ||
| - | As mentioned previously, the class I PI3Ks play a critical role in the transmission of proliferation and survival signals in a wide variety of cell types. Due to PI3Ks intricate activation system by numerous targets, mutations at key positions in PI3K have been identified to cause various types of [[Cancer|cancer]]. These positions are known as “Hotspots.” These hotspots are located in both the p85 subunit and p110 subunit. For example, a mutation in the <scene name='User:David_Canner/Sandbox_P/Med_nsh2_overview/1'>nSH2 domain</scene> known to cause glioblastoma is G376R. <scene name='User:David_Canner/Sandbox_P/Med_376/1'>Gly 376 is at hydrogen bonding distance of Glu 365 </scene> a crucial residue in one of the stabilizing C2 domain loops. <ref name="Amzel"/> Somatic mutations in the gene encoding the p110 catalytic subunit can be grouped into the four classes of the catalytic subunit in which they occur, the ABD, C2, helical and catalytic domains, all of which likely increase PI3K activity by different mechanisms.<ref name="Miled"/> For example, two well known cancer causing mutations map to <scene name='User:David_Canner/Sandbox_P/Helical_abd_out/2'>the ABD</scene>, at residues <scene name='User:David_Canner/Sandbox_P/Helical_abd_mutations/1'>Arg 38, Arg 88</scene>. These residues lie at the interface of the ABD and Kinase domains and are believed to alter regulation of the catalytic subunit. Other mutations, such as those in the C2 domain, up regulate PI3K, by increasing the affinity for substrate containing membranes, resulting in elevated levels of PIP3. <ref name="Miled"/> Aberrations in PIP3 levels, either through activation of PI3Ks or through inactivation of lipid phosphatase PTEN, occur frequently in numerous forms of cancer. Recent data suggest that at least 50% of human breast cancers involve mutations in either PI3K or [[PTEN]]. <ref name="Miled"/> | ||
| - | |||
| - | The dramatic number of mutations in PI3K associated with [[Cancer]] has resulted in PIK3CA, the gene that encodes the catalytic p100( domain of PIK3, being identified as a human [[oncogene]]. More than 1500 PIK3CA mutations, nearly all of which increase lipid kinase activity, have been identified in different tumor types, the most common being breast and uterine cancers. <ref name="Amzel"/> Further, the lipid products of PI3K interact with other well known oncogenes like akt2, akt3, PDGFR, [[PTEN]], among many others. <ref name="Walker"/> | ||
| - | |||
| - | In addition to [[cancer]], faulty PI3K function has been associated with disorders like heart failure <ref>PMID:20237330</ref>, [[Diabetes|diabetes]], <ref>PMID:18794886</ref>, and inflammation.<ref name="Fubar"/> | ||
| - | |||
| - | ==Current Pharmaceutical Approaches== | ||
| - | Broad spectrum PI3K inhibitors have exhibited impressive results, revealing increased apoptosis and decreased proliferation in tumor models.<ref name="Miled"/> The primary focus now amongst medicinal researchers is to identify PI3K inhibitors with increased selectivity (particularly for p110) and bioavailability. Use of inhibitors such as wortmannin have identified slightly different binding mechanisms between PI3K isoforms, creating the potential for highly selective compounds to neutralize secific PI3K isotypes while leaving other forms of the ubiquitous protein unaltered. <ref>PMID:17371252</ref> | ||
| - | </StructureSection> | ||
| - | |||
| - | ==Additional 3D Structures== | ||
| - | Solved Structures of PI3K | ||
| - | |||
| - | ===Class I PI3K=== | ||
| - | ====PI3K SH2 Domain==== | ||
| - | [[2iug]], [[2iuh]], [[2iui]] – Crystal Structure of PI3K nSH2 Domain with Peptides <br/> | ||
| - | [[1h9o]] – Crystal Structure of PI3K SH2 Domain with PDGFR Peptide <br /> | ||
| - | [[1fu5]], [[1fu6]] – NMR structure of nSH2 Domain from PI3K <br /> | ||
| - | |||
| - | ====PI3K ISH2 Domain==== | ||
| - | [[3mtt]] – Crystal Structure of PI3K ISH2 Beta Crystal <br /> | ||
| - | [[3l4q]] – Crystal Structure of PI3K ISH2 in Influenza <br /> | ||
| - | [[2v1y]] – Crystal Structure of ISH2 in complex with ADB <br /> | ||
| - | |||
| - | ====PI3K SH3 Domain==== | ||
| - | [[3i5s]], [[3i5r]] – Crystal Structure of SH3 Domain in complex with peptide <br /> | ||
| - | [[2kt1]] – Crystal Structure of SH3 Domain in p85 beta <br /> | ||
| - | [[1pht]] – Crystal Structure of PI3K Alpha SH3 Domain <br /> | ||
| - | [[1pks]], [[1pkt]] – Crystal Structure of PI3K SH3 Domain <br /> | ||
| - | |||
| - | ====p110 Subunit of PI3K==== | ||
| - | [[3lj3]] – Crystal Structure of PI3K Gamma bound to Pyrrolopyridine-Benzofuran Inhibitor <br /> | ||
| - | [[3l54]] – Crystal Structure of PI3K Gamma <br /> | ||
| - | [[3l13]], [[3l16]], [[3l17]] – Crystal Structure of Pan-PI3-Kinase with Inhibitor <br /> | ||
| - | [[3l08]] – Crystal Structure of PI3K Gamma bound to GSK2126458 <br /> | ||
| - | [[3ibe]] – Crystal Structure of PI3K Gamma bound to Pyrazolopyrimidine Inhibitor <br /> | ||
| - | [[3hhm]], [[3hiz]] – Crystal Structure of p110 & NISH2 <br /> | ||
| - | [[3ene]] – Crystal Structure of PI3K Gamma with inhibitor <br /> | ||
| - | [[3dpd]] – Crystal Structure of PI3K with oxazines inhibitor <br /> | ||
| - | [[3dbs]] – Crystal Structure of PI3K Gamma bound to GDC0941 <br/ > | ||
| - | [[3csf]], [[3cst]] – Crystal Structure of p110 Gamma bound to organourethenium inhibitor <br /> | ||
| - | [[2x38]] – Crystal Structure of p110 Delta bound to IC87114 <br /> | ||
| - | [[2wxf]], [[2wxg]], [[2wxh]], [[2wxi]], [[2wxj]], [[2wxk]], [[2wxl]], [[2wxm]], [[2wxn]], [[2wxo]], [[2wxp]], [[2wxq]], [[2wxr]] – Crystal Structure of p110 Delta with Inhibitors <br /> | ||
| - | [[2v4l]] – Crystal Structure of PI3K p110 Gamma with inhibitor <br /> | ||
| - | [[2rd0]] – Crystal Structure of PI3K p110/p85 complex <br /> | ||
| - | [[2chw]], [[2chx]], [[2chz]] – Crystal Structure of PI3K Gamma with PIK-39 Inhibitor <br/ > | ||
| - | [[2a4z]], [[2a5u]] – Crystal Structure of PI3K gamma complex with AS604850 and AS605240 Inhibitors <br /> | ||
| - | [[1he8]] – Crystal Structure of RAS – PI3K Gamma Complex <br /> | ||
| - | [[1e7u]], [[1e7v]], [[1e7w]], [[1e7y]], [[1e7z]], [[1e90]], [[1e8x]] – Crystal Structure of PI3K Bound to Various Inhibitors <br /> | ||
| - | |||
| - | ====PI3K C2 Domain==== | ||
| - | [[2wwe]] – Crystal Structure of PI3K C2 Gamma Domain <br /> | ||
| - | [[2enq]] – Crystal Structure of C2 Domain, p110 Alpha <br /> | ||
| - | |||
| - | ===Class III PI3K=== | ||
| - | [[2x6f]], [[2x6h]], [[2x6j]], [[2x6k]] – Crystal Structure of Class III PI3K bound to various inhibitors <br /> | ||
| - | [[3ls8]] – Crystal Structure of Class III PI3K in complex with inhibitor <br /> | ||
| - | [[3ihy]] – Crystal Structure of Human PI3K Class III <br/> | ||
==Additional Resources== | ==Additional Resources== | ||
| Line 150: | Line 52: | ||
<references /> | <references /> | ||
| + | [[Category:Topic Page]] | ||
| + | [[Category:Featured in BAMBED]] | ||
__NOEDITSECTION__ | __NOEDITSECTION__ | ||
Current revision
This page, as it appeared on November 15, 2010, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
Additional Resources
- See: Cancer For Additional Proteins involved in the disease.
- See: Oncogenes for Additional examples of oncogenes and tumor suppressor genes.
References
- ↑ Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002 Aug;27(8):426-32. PMID:12151228
- ↑ 2.0 2.1 2.2 Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998 Dec 8;1436(1-2):127-50. PMID:9838078
- ↑ Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, Suzuki A, Bouchard D, Ho A, Redston M, Gallinger S, Khokha R, Mak TW, Hawkins PT, Stephens L, Scherer SW, Tsao M, Penninger JM. Colorectal carcinomas in mice lacking the catalytic subunit of PI(3)Kgamma. Nature. 2000 Aug 24;406(6798):897-902. PMID:10972292 doi:10.1038/35022585
- ↑ Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999 Apr 16;274(16):10963-8. PMID:10196176
- ↑ Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, Schneidman-Duhovny D, Wolfson HJ, Backer JM, Williams RL. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007 Jul 13;317(5835):239-42. PMID:17626883 doi:317/5835/239
- ↑ Stephens LR, Hughes KT, Irvine RF. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33-9. PMID:1851250 doi:http://dx.doi.org/10.1038/351033a0
- ↑ Hoedemaeker FJ, Siegal G, Roe SM, Driscoll PC, Abrahams JP. Crystal structure of the C-terminal SH2 domain of the p85alpha regulatory subunit of phosphoinositide 3-kinase: an SH2 domain mimicking its own substrate. J Mol Biol. 1999 Oct 1;292(4):763-70. PMID:10525402 doi:http://dx.doi.org/10.1006/jmbi.1999.3111
- ↑ Harris SJ, Foster JG, Ward SG. PI3K isoforms as drug targets in inflammatory diseases: lessons from pharmacological and genetic strategies. Curr Opin Investig Drugs. 2009 Nov;10(11):1151-62. PMID:19876783
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Joel L. Sussman, Jaime Prilusky, Hannah Campbell, Alexander Berchansky, Angel Herraez