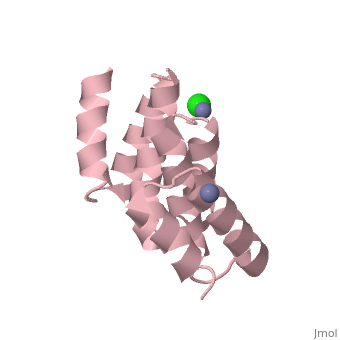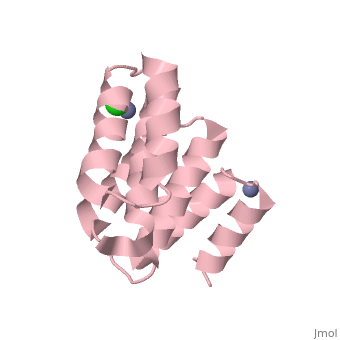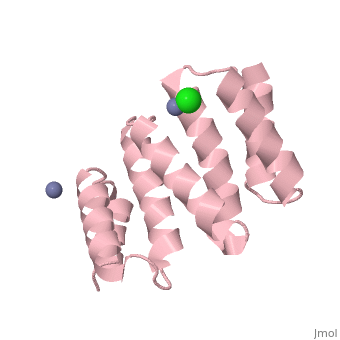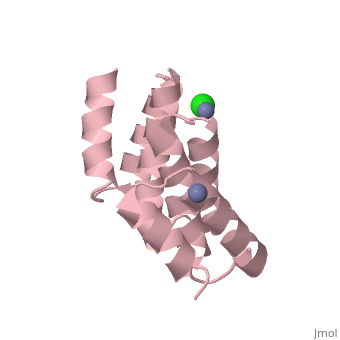We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
YbgF
From Proteopedia
(Difference between revisions)
| (20 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:YbgF.jpg| | + | <StructureSection load='2xev' size='350' side='right' scene='' caption='YbgF TPR domain complex with Zn+2 (grey) and Ca+2 (green) ions (PDB code [[2xev]])'> |
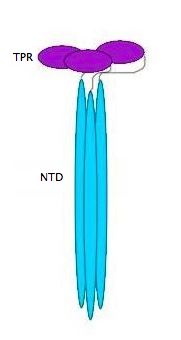
| - | {{ | + | [[Image:YbgF.jpg|200px|left|thumb| The Structure of YbgF<ref name='Gerding'>PMID: 17233825</ref> ]] |
| - | + | {{Clear}} | |
| + | __TOC__ | ||
==Structure== | ==Structure== | ||
| - | YbgF is a periplasmic protein with an N-terminal coiled coil domain (NTD) and a C-terminal tetratricopeptide domain (TPR), both of which are autonomous<ref name='Gerding'>PMID: 17233825</ref>. As seen in the 3D structure 2WZ7, the NTD forms an elongated trimer which is connected via a flexible linker to the TPR trimer, as seen in 2XEV. This connection can be cleaved by proteases<ref name='Krachler'>PMID: 20816983</ref>. | + | '''YbgF''' is a periplasmic protein with an N-terminal coiled coil domain (NTD) and a C-terminal tetratricopeptide domain (TPR), both of which are autonomous<ref name='Gerding'>PMID: 17233825</ref>. As seen in the <scene name='42/429024/Cv/1'>3D structure 2WZ7</scene>, the NTD forms an elongated trimer which is connected via a flexible linker to the TPR trimer, <scene name='42/429024/Cv/2'>as seen in 2XEV</scene>. This connection can be cleaved by proteases<ref name='Krachler'>PMID: 20816983</ref>. |
==Function== | ==Function== | ||
| Line 12: | Line 13: | ||
Studies have shown that inactivation of the YbgF gene results in no discernible change in the activity of Tol<ref name='Walberger'>PMID: 11994151</ref>. Therefore, replacing the protein with another would not rescue any lost function. Future research may look into comparison of different gram negative bacteria in order to determine if the function is conserved. One particular organism that is known to not have this domain conserved is ''Chlamydiae''<ref name='Krachler'>PMID: 20816983</ref>. Future research may look into the functioning of its Tol system, with a the possible addition of the YbgF domain to study what effect this might have. | Studies have shown that inactivation of the YbgF gene results in no discernible change in the activity of Tol<ref name='Walberger'>PMID: 11994151</ref>. Therefore, replacing the protein with another would not rescue any lost function. Future research may look into comparison of different gram negative bacteria in order to determine if the function is conserved. One particular organism that is known to not have this domain conserved is ''Chlamydiae''<ref name='Krachler'>PMID: 20816983</ref>. Future research may look into the functioning of its Tol system, with a the possible addition of the YbgF domain to study what effect this might have. | ||
| + | ==3D structures of Ybgf== | ||
| + | |||
| + | [[2wz7]], [[2xdj]] – Ybgf N terminal – ''Escherichia coli''<br /> | ||
| + | [[2xev]] – Ybgf TPR domain – ''Xanthomonas campestris'' | ||
| + | |||
| + | </StructureSection> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Gerding MA, Ogata Y, Pecora ND, Niki H, de Boer PA. The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol Microbiol. 2007 Feb;63(4):1008-25. PMID:17233825 doi:10.1111/j.1365-2958.2006.05571.x
- ↑ 2.0 2.1 2.2 2.3 Krachler AM, Sharma A, Cauldwell A, Papadakos G, Kleanthous C. TolA modulates the oligomeric status of YbgF in the bacterial periplasm. J Mol Biol. 2010 Oct 22;403(2):270-85. Epub 2010 Sep 15. PMID:20816983 doi:10.1016/j.jmb.2010.08.050
- ↑ 3.0 3.1 Walburger A, Lazdunski C, Corda Y. The Tol/Pal system function requires an interaction between the C-terminal domain of TolA and the N-terminal domain of TolB. Mol Microbiol. 2002 May;44(3):695-708. PMID:11994151
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Laura McCauley, Joel L. Sussman, Alexander Berchansky