We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Aldolase
From Proteopedia
(Difference between revisions)
| (58 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
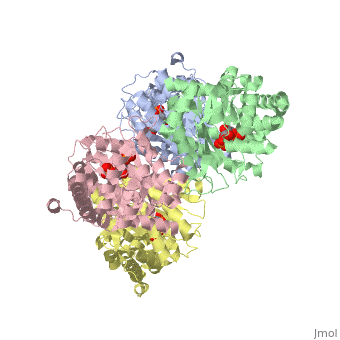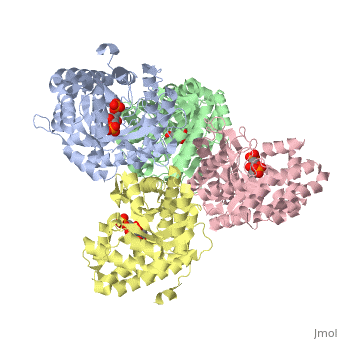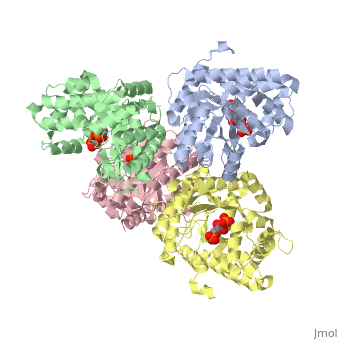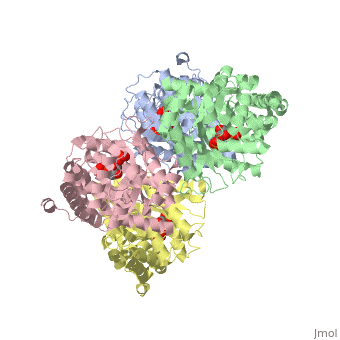
| + | <StructureSection load='3mmt' size='340' side='right' caption='Fructose 1,6-bisphosphate aldolase tetramer complex with fructose 1,6-bisphosphate, [[3mmt]]|' scene=''> | ||
| + | '''Retro aldolase''' is an aldolase designed by directed evolution. | ||
| + | =Aldolase class I= | ||
| + | |||
| + | '''Fructose-6-phosphate aldolase''' catalyzes the cleavage of fructose-6-phosphate<ref>PMID:11120740</ref>.<br /> | ||
| + | '''Fructose-1,6-bisphosphate aldolase''' (FBPA) catalyzes the fourth step of glycolysis<ref>PMID:34458323</ref>.<br /> | ||
| + | '''Deoxyribose-phosphate aldolase''' converts 2-deoxy-D-ribose-5-phosphate into glyceraldehyde 3-phosphate and acetaldehyde<ref>PMID:25229427</ref>.<br /> | ||
| + | '''Dihydroneopterin aldolase''' catalyzes the conversion of 7,8-dihydropterin to 6-hydroxymethyl-7,8-dihydropterin. It is part of the folate synthesis <ref>PMID:15107504</ref>.<br /> | ||
| + | '''Sialic acid aldolase''' catalyzes the condensation of pyruvate and N-acetylmannosamine<ref>PMID:11674166</ref>. See [[N-acetylneuraminate lyase]].<br /> | ||
| + | '''Oxoadipate aldolase''' catalyzes the last step of the bacterial protocatechuate 4,5-cleavage pathway<ref>PMID:20843800</ref>.<br /> | ||
| + | '''Oxovalerate aldolase''' catalyzes the conversion of 4-hydroxy-2-oxopentanoate to acetaldehyde and pyruvate<ref>PMID:8419288</ref>.<br /> | ||
| + | '''2-keto-deoxydephosphogluconate aldolase''' catalyzes the cleavage of 2-keto-deoxydephosphogluconate<ref>PMID:12876349</ref>.<br /> | ||
| + | '''Phospho-2-dehydro-3-deoxyheptonate aldolase''' participates in the phthalide biosynthesis<ref>PMID:35845641</ref>.<br /> | ||
| + | =Aldolase class II. Metal-dependent aldolase= | ||
| + | |||
| + | '''Fructose-1,6-bisphosphate aldolase''' catalyzes the conversion of fructose-1,6-bisphosphatealdol to dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P) <ref>PMID:10712619</ref>.<br /> | ||
| + | '''Tagatose-1,6-bisphosphate aldolase''' catalyzes the aldol condensation of DHAP with G3P to produce tagatose 1,6-bisphosphate<ref>PMID:11940603</ref>.<br /> | ||
| + | '''Fuculose-1-phosphate aldolase''' catalyzes the cleavage of fuculose-1-phosphate to dihydroxyacetone phosphate (DHAP) and lactaldehyde<ref>PMID:10821675</ref>.<br /> | ||
| + | '''HpcH/HpaI aldolase''' catalyzes the conversion of 4-hydroxy-2-oxo-heptane-1,7-dioate into pyruvate and succinate. It is part of the aromatic compounds degradation<ref>PMID:17881002</ref>.<br /> | ||
| + | '''Oxoglutarate aldolase''' catalyzes the cleavage of 4-hydroxy-2-oxoglutarate into pyruvate and glyoxylate. It belongs to the hydroxyproline degradation pathway<ref>PMID:21998747</ref>.<br /> | ||
| + | '''Threonine aldolase''' catalyzes the cleavage of threonine into glycine and acetaldehyde. It is part of the glycine, serine and threonine metabolism pathway<ref>PMID:13449064</ref>.<br /> | ||
| + | '''Rhamnulose-1-phosphate aldolase''' participates in the degradation pathway of L-rhamnose <ref>PMID:18085797</ref>.<br /> | ||
| + | '''4-hydroxy-2-oxoglutarate aldolase''' catalyzes the cleavage of 4-hydroxy-2-oxoglutarate to pyruvate and glyoxylate It is part of the hydroxyproline degradation pathway<ref>PMID:21998747</ref>.<br /> | ||
| + | '''Hydroxyaspartate aldolase''' catalyzes the cleavage of hydroxyaspartate to glyoxylate and glycine<ref>PMID:12835921</ref>.<br /> | ||
| + | '''Phenylserine aldolase''' catalyzes the cleavage of L-3-phenylserine to benzaldehyde and glycine<ref>PMID:16085854</ref>.<br /> | ||
= Fructose Bisphosphate Aldolase = | = Fructose Bisphosphate Aldolase = | ||
| - | {{STRUCTURE_4ald | PDB=4ald | SCENE= }} | ||
==Introduction and Structure== | ==Introduction and Structure== | ||
| + | '''Fructose bisphosphate aldolase''' is an enzyme in glycolysis and gluconeogenesis. Glycolyis is responsible for the conversion of glucose into two three-carbon pyruvate molecules without the need for oxygen. The process generates two net ATP. The overall reaction is: | ||
| + | Glucose + 2 NAD+ + 2 ADP + 2 Pi --> 2 pyruvate (3-carbon product) + 2 NADH + 2 ATP + 2 H<sub>2</sub>0 + 4 H+ | ||
| - | + | Gluconeogenesis is responsible for maintaining the appropriate levels of blood glucose in animals by generating glucose from non-carbohydrate precursors. Gluconeogenesis can make glucose from lactate, pyruvate, citric acid cycle intermediates and from most amino acids (the exceptions being leucine and lysine). The common intermediate for all of the precursors on their way to becoming glucose must be oxaloacetate. | |
| - | + | The aldolase catalyzes the reversible cleavage of fructose-1,6-bisphosphate into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP). Different isozymes of aldolase can also catalyze the cleavage of fructose 1-phosphate to diydroxyacetone and glyceraldehyde (GA). Different isozymes exhibit preferences for either or both of the substrates, depending on the role of the aldolase (i.e. gluconeogenesis versus glycolysis).<ref name="book">Voet, D, Voet, J, & Pratt, C. (2008). Fundamentals of biochemistry, third edition. Hoboken, NJ: Wiley & Sons, Inc.</ref> See also [[Enzimas: complejo enzima-sustrato]] (in Spanish) and [[Glycolysis Enzymes]]. | |
| - | + | <!-- | |
| - | + | <StructureSection load='4ald' size='350' frame='true' align='right' scene='Aldolase/Cv/1' caption='Monomer of the tetrameric fructose 1,6-bisphosphate aldolase complex with fructose 1,6-bisphosphate, [[4ald]]'> --> | |
| - | + | ||
While it can exist as a monomer, it normally exists as a <scene name='Austin_Drake_Sandbox/Tetramer/3'>homotetramer</scene>. The enzyme is an a/B protein with a TIM beta/alpha beta fold. The fold designation is based upon the nine alpha helices and eight parallel beta sheets in a closed barrel of each monomeric subunit. It is part of the aldolase superfamily and the class I aldolases.<ref>Protein: fructose-1,6-bisphosphate aldolase from human (homo sapiens), muscle isozyme. (2009). Retrieved from http://scop.mrc-lmb.cam.ac.uk</ref> <scene name='Austin_Drake_Sandbox/Different_colors/3'>α helices and β sheets</scene> can be seen in their specific regions mostly concentric to the active site, represented by the blue and red residues. | While it can exist as a monomer, it normally exists as a <scene name='Austin_Drake_Sandbox/Tetramer/3'>homotetramer</scene>. The enzyme is an a/B protein with a TIM beta/alpha beta fold. The fold designation is based upon the nine alpha helices and eight parallel beta sheets in a closed barrel of each monomeric subunit. It is part of the aldolase superfamily and the class I aldolases.<ref>Protein: fructose-1,6-bisphosphate aldolase from human (homo sapiens), muscle isozyme. (2009). Retrieved from http://scop.mrc-lmb.cam.ac.uk</ref> <scene name='Austin_Drake_Sandbox/Different_colors/3'>α helices and β sheets</scene> can be seen in their specific regions mostly concentric to the active site, represented by the blue and red residues. | ||
| Line 22: | Line 47: | ||
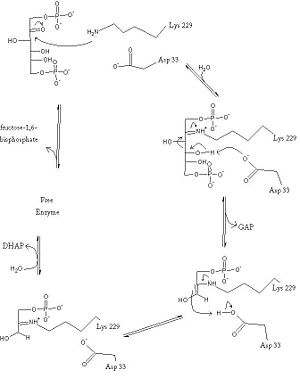
The reaction is an aldol cleavage, or otherwise termed, retro aldo condensation. Catalysis occurs first when the nucleophilic ε-amine group of Lys229 attacks the carbonyl carbon of the substrate (FBP) in its open-ring state, pushing an electron pair to the oxygen of the carbonyl. The oxygen is protonated and leaves as water as a protonated <scene name='Austin_Drake_Sandbox/Schiff_base/2'>Schiff base</scene> is produced (an imine resulting from a ketone and amine) with the open-ring form of FBP, accompanied by electrostatic stabilization from <scene name='Austin_Drake_Sandbox/Catalytic_site_w_water/5'>Asp33</scene> Aldol cleavage between C3 and C4 produces GAP and an enamine precursor to DHAP.<ref name="book" /> The cleavage is facilitated by the positive charge from the Schiff base. The subsequent electron movement, which alleviates the positive charge, also breaks the C3-C4 bond.<ref name="review" /> Tautomerization, protonation and the hydrolysis of the Schiff base produce the final product of DHAP and regenerate the enzyme. The catalysis is driven by the more favorable stability of the protonated Schiff base compared to the enolate that would appear in basic catalysis pathways.<ref name="book" /> | The reaction is an aldol cleavage, or otherwise termed, retro aldo condensation. Catalysis occurs first when the nucleophilic ε-amine group of Lys229 attacks the carbonyl carbon of the substrate (FBP) in its open-ring state, pushing an electron pair to the oxygen of the carbonyl. The oxygen is protonated and leaves as water as a protonated <scene name='Austin_Drake_Sandbox/Schiff_base/2'>Schiff base</scene> is produced (an imine resulting from a ketone and amine) with the open-ring form of FBP, accompanied by electrostatic stabilization from <scene name='Austin_Drake_Sandbox/Catalytic_site_w_water/5'>Asp33</scene> Aldol cleavage between C3 and C4 produces GAP and an enamine precursor to DHAP.<ref name="book" /> The cleavage is facilitated by the positive charge from the Schiff base. The subsequent electron movement, which alleviates the positive charge, also breaks the C3-C4 bond.<ref name="review" /> Tautomerization, protonation and the hydrolysis of the Schiff base produce the final product of DHAP and regenerate the enzyme. The catalysis is driven by the more favorable stability of the protonated Schiff base compared to the enolate that would appear in basic catalysis pathways.<ref name="book" /> | ||
| - | + | [[Image:Aldolase1.jpg|border|300px]] | |
| - | [[Image:Aldolase1.jpg]] | + | |
==Kinetics== | ==Kinetics== | ||
| Line 33: | Line 57: | ||
==Regulation== | ==Regulation== | ||
| - | The regulation of fructose 1,6-bisphosphate aldolase is not well understood, but the understanding is | + | The regulation of fructose 1,6-bisphosphate aldolase is not well understood, but the understanding is ever-increasing. As it is currently observed, aldolase C appears to be regulated mainly by the gene expression--the concentration of mRNA in the cytoplasm.<ref>Paolella, G, Buono, P, Mancini, F P, Izzo, P, and Salvatore, F. "Structure and expression of mouse aldolase genes." Eur. J. Biochem.. 156. (1986): 229-235.</ref> It is also known that adenosine 3',5'-cyclicmonophosphate (cAMP) affects the expression of the gene. cAMP concentration has been positively correlated with aldolase C expression. It is believed that cAMP acts upon a section of the promotor region, distal element D, causing the transcriptional promoter, NGFI-B, to bind. Once bound, the promoter activates the transcription of the gene coding for fructose bisphosphate aldolase.<ref>Buono, P, Cassano, S, Alfieri, A, Mancini, A, and Salvatore, F. "Human aldolase C gene expression is regulated by adenosine 30,50-cyclic monophosphate (cAMP) in PC12 cells." Gene. 291. (2002): 115-121.</ref> Given the inhibitory effects of an oxidant in the presence of aldolase, it is possible that this could be a mechanism of regulation of the enzyme. The deactivation that accompanies the oxidation of the surface thiol of Cys72 could be used intracellularly to slow the catalysis of the enzyme and regulate glycolysis.<ref name="kinetics" /> |
| - | + | =3D structures of aldolase= | |
| + | [[Aldolase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | =Additional Resources= | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
For additional information, see: [[Carbohydrate Metabolism]] | For additional information, see: [[Carbohydrate Metabolism]] | ||
<br /> | <br /> | ||
| - | + | =References= | |
<references/> | <references/> | ||
| + | |||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Schurmann M, Sprenger GA. Fructose-6-phosphate aldolase is a novel class I aldolase from Escherichia coli and is related to a novel group of bacterial transaldolases. J Biol Chem. 2001 Apr 6;276(14):11055-61. Epub 2000 Dec 18. PMID:11120740 doi:http://dx.doi.org/10.1074/jbc.M008061200
- ↑ Pirovich DB, Da'dara AA, Skelly PJ. Multifunctional Fructose 1,6-Bisphosphate Aldolase as a Therapeutic Target. Front Mol Biosci. 2021 Aug 11;8:719678. PMID:34458323 doi:10.3389/fmolb.2021.719678
- ↑ Salleron L, Magistrelli G, Mary C, Fischer N, Bairoch A, Lane L. DERA is the human deoxyribose phosphate aldolase and is involved in stress response. Biochim Biophys Acta. 2014 Dec;1843(12):2913-25. doi:, 10.1016/j.bbamcr.2014.09.007. Epub 2014 Sep 16. PMID:25229427 doi:http://dx.doi.org/10.1016/j.bbamcr.2014.09.007
- ↑ Goyer A, Illarionova V, Roje S, Fischer M, Bacher A, Hanson AD. Folate biosynthesis in higher plants. cDNA cloning, heterologous expression, and characterization of dihydroneopterin aldolases. Plant Physiol. 2004 May;135(1):103-11. Epub 2004 Apr 23. PMID:15107504 doi:http://dx.doi.org/10.1104/pp.103.038430
- ↑ Smith BJ, Lawrence MC, Barbosa JA. Substrate-Assisted Catalysis in Sialic Acid Aldolase. J Org Chem. 1999 Feb 5;64(3):945-949. PMID:11674166
- ↑ Wang W, Mazurkewich S, Kimber MS, Seah SY. Structural and kinetic characterization of 4-hydroxy-4-methyl-2-oxoglutarate (HMG)/4-carboxy-4-hydroxy-2-oxoadipate (CHA) aldolase: a protocatechuate degradation enzyme evolutionarily convergent with the HpaI and DmpG pyruvate aldolases. J Biol Chem. 2010 Sep 15. PMID:20843800 doi:10.1074/jbc.M110.159509
- ↑ Powlowski J, Sahlman L, Shingler V. Purification and properties of the physically associated meta-cleavage pathway enzymes 4-hydroxy-2-ketovalerate aldolase and aldehyde dehydrogenase (acylating) from Pseudomonas sp. strain CF600. J Bacteriol. 1993 Jan;175(2):377-85. PMID:8419288
- ↑ Bell BJ, Watanabe L, Rios-Steiner JL, Tulinsky A, Lebioda L, Arni RK. Structure of 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase from Pseudomonas putida. Acta Crystallogr D Biol Crystallogr. 2003 Aug;59(Pt 8):1454-8. Epub 2003, Jul 23. PMID:12876349
- ↑ Feng WM, Liu P, Yan H, Yu G, Zhang S, Jiang S, Shang EX, Qian DW, Duan JA. Investigation of Enzymes in the Phthalide Biosynthetic Pathway in Angelica sinensis Using Integrative Metabolite Profiles and Transcriptome Analysis. Front Plant Sci. 2022 Jul 1;13:928760. PMID:35845641 doi:10.3389/fpls.2022.928760
- ↑ Zgiby SM, Thomson GJ, Qamar S, Berry A. Exploring substrate binding and discrimination in fructose1, 6-bisphosphate and tagatose 1,6-bisphosphate aldolases. Eur J Biochem. 2000 Mar;267(6):1858-68. PMID:10712619
- ↑ Hall DR, Bond CS, Leonard GA, Watt CI, Berry A, Hunter WN. Structure of tagatose-1,6-bisphosphate aldolase. Insight into chiral discrimination, mechanism, and specificity of class II aldolases. J Biol Chem. 2002 Jun 14;277(24):22018-24. Epub 2002 Apr 8. PMID:11940603 doi:http://dx.doi.org/10.1074/jbc.M202464200
- ↑ Joerger AC, Gosse C, Fessner WD, Schulz GE. Catalytic action of fuculose 1-phosphate aldolase (class II) as derived from structure-directed mutagenesis. Biochemistry. 2000 May 23;39(20):6033-41. PMID:10821675
- ↑ Rea D, Fulop V, Bugg TD, Roper DI. Structure and mechanism of HpcH: a metal ion dependent class II aldolase from the homoprotocatechuate degradation pathway of Escherichia coli. J Mol Biol. 2007 Nov 2;373(4):866-76. Epub 2007 Jun 26. PMID:17881002 doi:10.1016/j.jmb.2007.06.048
- ↑ Riedel TJ, Johnson LC, Knight J, Hantgan RR, Holmes RP, Lowther WT. Structural and Biochemical Studies of Human 4-hydroxy-2-oxoglutarate Aldolase: Implications for Hydroxyproline Metabolism in Primary Hyperoxaluria. PLoS One. 2011;6(10):e26021. Epub 2011 Oct 6. PMID:21998747 doi:10.1371/journal.pone.0026021
- ↑ KARASEK MA, GREENBERG DM. Studies on the properties of threonine aldolases. J Biol Chem. 1957 Jul;227(1):191-205. PMID:13449064
- ↑ Grueninger D, Schulz GE. Antenna domain mobility and enzymatic reaction of L-rhamnulose-1-phosphate aldolase. Biochemistry. 2008 Jan 15;47(2):607-14. Epub 2007 Dec 18. PMID:18085797 doi:http://dx.doi.org/10.1021/bi7012799
- ↑ Riedel TJ, Johnson LC, Knight J, Hantgan RR, Holmes RP, Lowther WT. Structural and Biochemical Studies of Human 4-hydroxy-2-oxoglutarate Aldolase: Implications for Hydroxyproline Metabolism in Primary Hyperoxaluria. PLoS One. 2011;6(10):e26021. Epub 2011 Oct 6. PMID:21998747 doi:10.1371/journal.pone.0026021
- ↑ Liu JQ, Dairi T, Itoh N, Kataoka M, Shimizu S. A novel enzyme, D-3-hydroxyaspartate aldolase from Paracoccus denitrificans IFO 13301: purification, characterization, and gene cloning. Appl Microbiol Biotechnol. 2003 Jul;62(1):53-60. PMID:12835921 doi:10.1007/s00253-003-1238-2
- ↑ Misono H, Maeda H, Tuda K, Ueshima S, Miyazaki N, Nagata S. Characterization of an inducible phenylserine aldolase from Pseudomonas putida 24-1. Appl Environ Microbiol. 2005 Aug;71(8):4602-9. PMID:16085854 doi:10.1128/AEM.71.8.4602-4609.2005
- ↑ 20.0 20.1 20.2 Voet, D, Voet, J, & Pratt, C. (2008). Fundamentals of biochemistry, third edition. Hoboken, NJ: Wiley & Sons, Inc.
- ↑ Protein: fructose-1,6-bisphosphate aldolase from human (homo sapiens), muscle isozyme. (2009). Retrieved from http://scop.mrc-lmb.cam.ac.uk
- ↑ 22.0 22.1 22.2 Gefflaut, T., B. Casimir, J. Perie, and M. Willson. "Class I Aldolases: Substrate Specificity, Mechanism, Inhibitors and Structural Aspects." Prog. Biophys. molec. Biol.. 63. (1995): 301-340.
- ↑ Dalby A, Dauter Z, Littlechild JA. Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate: mechanistic implications. Protein Sci. 1999 Feb;8(2):291-7. PMID:10048322
- ↑ 24.0 24.1 Sygusch, J., and Beaudry, D. "Allosteric communication in mammalian muscle aldolase." Biochem. J.. 327. (1997): 717-720.
- ↑ Paolella, G, Buono, P, Mancini, F P, Izzo, P, and Salvatore, F. "Structure and expression of mouse aldolase genes." Eur. J. Biochem.. 156. (1986): 229-235.
- ↑ Buono, P, Cassano, S, Alfieri, A, Mancini, A, and Salvatore, F. "Human aldolase C gene expression is regulated by adenosine 30,50-cyclic monophosphate (cAMP) in PC12 cells." Gene. 291. (2002): 115-121.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Sophie Mullinix, Jaime Prilusky, Austin Drake, David Canner