We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
RTP and Tus
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='' size='450' side='right' scene='RTP_and_Tus/Practice_structure/2' caption=''> | ||
| + | __NOTOC__ | ||
A comparison of the Replication Terminator Protein (from ''Bacillus subtilis'') and Tus (from ''Escherichia coli'') provides an interesting insight into how proteins with vastly different structures and mechanisms of action can produce essentially identical effects in their native systems. | A comparison of the Replication Terminator Protein (from ''Bacillus subtilis'') and Tus (from ''Escherichia coli'') provides an interesting insight into how proteins with vastly different structures and mechanisms of action can produce essentially identical effects in their native systems. | ||
Looking at the structures of these two proteins, it is not immediately obvious that they would perfom the same function; to arrest the progression of the replication fork along the bacterial chromosome at specific sites (''Ter'' sites). Furthermore, this arrest-mechanism functions in a polar manner in both organisms, which is perhaps surprising considering the symmetrical characteristics of both proteins.<ref>Wake, RG and King, GF (1997) A tale of two terminators: crystal structures sharpen the debate on DNA replication fork arrest mechanisms. ''Structure'' 5: 1-5.</ref> | Looking at the structures of these two proteins, it is not immediately obvious that they would perfom the same function; to arrest the progression of the replication fork along the bacterial chromosome at specific sites (''Ter'' sites). Furthermore, this arrest-mechanism functions in a polar manner in both organisms, which is perhaps surprising considering the symmetrical characteristics of both proteins.<ref>Wake, RG and King, GF (1997) A tale of two terminators: crystal structures sharpen the debate on DNA replication fork arrest mechanisms. ''Structure'' 5: 1-5.</ref> | ||
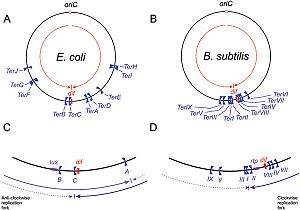
| - | [[Image:Replication_fork.jpg|300px| | + | [[Image:Replication_fork.jpg|300px|left|thumb| Schematic representation of ''Ter'' sites in ''B. subtilis'' and ''E. coli'', Taken from Duggin ''et al'' (2008). ]] |
| - | + | {{Clear}} | |
| - | + | ||
== The Replication Fork and Polar Arrest == | == The Replication Fork and Polar Arrest == | ||
| Line 11: | Line 12: | ||
DNA replication of circular bacterial chromosomes occurs using two replication forks that originate from a single location (''oriC'') and move in opposite directions around the chromosome. In ''E. coli'', ''B. subtilis'', and other bacteria and archaea, these replication forks are halted by interactions with terminator proteins bound to DNA sites known as "Terminator sites", or''Ter'' sites. The termination of the replication fork is dependent on the direction of approach to these ''Ter'' sites: if the replication fork approaches from the permissive face replication will continue; however, if the replication fork approaches from the non-permissive face the fork will be arrested and DNA replication will cease at that point. While it is possible for these organisms to function without this type of replication-arrest mechanism, the conservation of this system across species indicates some form of evolutionary benefit. | DNA replication of circular bacterial chromosomes occurs using two replication forks that originate from a single location (''oriC'') and move in opposite directions around the chromosome. In ''E. coli'', ''B. subtilis'', and other bacteria and archaea, these replication forks are halted by interactions with terminator proteins bound to DNA sites known as "Terminator sites", or''Ter'' sites. The termination of the replication fork is dependent on the direction of approach to these ''Ter'' sites: if the replication fork approaches from the permissive face replication will continue; however, if the replication fork approaches from the non-permissive face the fork will be arrested and DNA replication will cease at that point. While it is possible for these organisms to function without this type of replication-arrest mechanism, the conservation of this system across species indicates some form of evolutionary benefit. | ||
| + | [[Image:1BM9.2.jpg|400px|left|thumb| First determined RTP structure. By Bussiere ''et al.''(1995)]][[Image:1ECR.jpg|400px|left|thumb| Unlocked Tus-''Ter'' complex, detemined by Kamada ''et al.''(1996)]] | ||
| + | {{Clear}} | ||
| - | |||
| - | [[Image:1BM9.2.jpg|400px|left|thumb| First determined RTP structure. By Bussiere ''et al.''(1995)]][[Image:1ECR.jpg|400px|right|thumb| Unlocked Tus-''Ter'' complex, detemined by Kamada ''et al.''(1996)]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | <Structure load='1F4K' size='400' frame='true' align='left' caption='RTP complexed with ''Ter''DNA' (Wilce ''et al.'', 2001)' scene='RTP_and_Tus/Practice_structure/2' /> | ||
== RTP: A homodimer responsible for polar arrest == | == RTP: A homodimer responsible for polar arrest == | ||
| - | + | <scene name='44/448729/Practice_structure/8'>RTP complexed with TerDNA</scene> | |
| - | + | ||
The Replication Terminator Protein (RTP) from ''Bacillus subtilis'' is comprised of two identical monomers 14.5 kDa in size which bind to DNA to form a homodimer. The separate monomers bind at 30 bp sequences known as the A and B termination (''Ter'') sites. Both of these sites have inverted 16 bp repeats which overlap at highly conserved TAT trinucleotide sequence. The structure of RTP is commonly referred to as a “winged helix” DNA binding motif and consists of a compact α helix / β-strand <scene name='RTP_and_Tus/Practice_structure/5'>secondary structure</scene> with a protruding loop (or “wing”) between the β2 and β3 strands. Both monomers of RTP interact with DNA specifically through hydrogen bonding at residues | The Replication Terminator Protein (RTP) from ''Bacillus subtilis'' is comprised of two identical monomers 14.5 kDa in size which bind to DNA to form a homodimer. The separate monomers bind at 30 bp sequences known as the A and B termination (''Ter'') sites. Both of these sites have inverted 16 bp repeats which overlap at highly conserved TAT trinucleotide sequence. The structure of RTP is commonly referred to as a “winged helix” DNA binding motif and consists of a compact α helix / β-strand <scene name='RTP_and_Tus/Practice_structure/5'>secondary structure</scene> with a protruding loop (or “wing”) between the β2 and β3 strands. Both monomers of RTP interact with DNA specifically through hydrogen bonding at residues | ||
| Line 67: | Line 29: | ||
Early theories to explain replication fork arrest by RTP involved the concept of a “molecular clamp”, which suggests that the affinity between the ''Ter'' DNA and the RTP protein is sufficient to stop the replication fork. One of the earliest theories was the '''Induced Conformational Change''' Model. This model, first described by Smith ''et al.'' in 1996, proposes that when the first monomer of RTP binds the ''Ter''-B site on the chromosome it causes slight DNA bending, which promotes the binding of the second monomer of RTP to the A site via cooperative binding. According to this model, the binding of the second monomer to the A site changes conformation of ''Ter''-B-RTP complex such that it is able to inhibit DNA unwinding by the helicase enzyme and arrest the replication fork.<ref>Smith MT, de Vries CJ, Langley DB, King GF, Wake RG (1996) The ''Bacillis subtilis'' DNA Replication Terminator. ''Journal of Molecular Biology'' 260: 54-69.</ref> | Early theories to explain replication fork arrest by RTP involved the concept of a “molecular clamp”, which suggests that the affinity between the ''Ter'' DNA and the RTP protein is sufficient to stop the replication fork. One of the earliest theories was the '''Induced Conformational Change''' Model. This model, first described by Smith ''et al.'' in 1996, proposes that when the first monomer of RTP binds the ''Ter''-B site on the chromosome it causes slight DNA bending, which promotes the binding of the second monomer of RTP to the A site via cooperative binding. According to this model, the binding of the second monomer to the A site changes conformation of ''Ter''-B-RTP complex such that it is able to inhibit DNA unwinding by the helicase enzyme and arrest the replication fork.<ref>Smith MT, de Vries CJ, Langley DB, King GF, Wake RG (1996) The ''Bacillis subtilis'' DNA Replication Terminator. ''Journal of Molecular Biology'' 260: 54-69.</ref> | ||
| - | |||
| - | |||
| - | |||
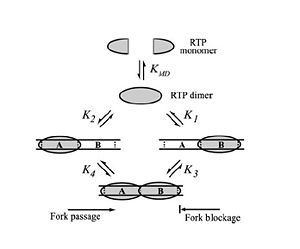
'''Differential Binding Affinity''' | '''Differential Binding Affinity''' | ||
| Line 75: | Line 34: | ||
[[Image:RTP_DBA_Model2.jpg|300px|right|thumb| Differential Binding Affinity Model proposed by Kralicek ''et al.'' in 1997. | [[Image:RTP_DBA_Model2.jpg|300px|right|thumb| Differential Binding Affinity Model proposed by Kralicek ''et al.'' in 1997. | ||
Image from Duggin ''et al.'', 2004. ]] | Image from Duggin ''et al.'', 2004. ]] | ||
| + | {{Clear}} | ||
In 1997, Kralicek ''et al.'' proposed an alternate theory of RTP arrest known as the '''Differential Binding Affinity''' Model. This model also involves the idea of a “molecular clamp”, and states that the polar arrest mechanism can be explained purely based on the differential binding affinities of RTP to the A and B termination sites. The theory is based on the assumption that the affinity of RTP for the B site in the complete complex is much greater than the affinity of for the A site in the complete complex, or the affinity of a single RTP monomer to the B site alone. According to the model, only the affinity of the complex RTP for the ''Ter''-B (K3 in Figure X) is sufficient to prevent the removal of RTP from DNA when the replication fork moves along the DNA; therefore, if the replisome approaches from the B site, the RTP is not removed from the DNA and the replication fork is arrested. Similarly, when the replication fork approaches from the A site the binding affinity (K4 in Figure X) is not sufficient to prevent the removal of RTP and the replisome is able to pass.<ref>Kralicek, AV, Wilson PK, Ralston GB, Wake RG, King GF (1997) Reorganization of terminator DNA upon binding replication terminator protein: implications for the functional replication fork arrest complex. ''Nucleic Acids Research'' 25(3): 590-596.</ref> | In 1997, Kralicek ''et al.'' proposed an alternate theory of RTP arrest known as the '''Differential Binding Affinity''' Model. This model also involves the idea of a “molecular clamp”, and states that the polar arrest mechanism can be explained purely based on the differential binding affinities of RTP to the A and B termination sites. The theory is based on the assumption that the affinity of RTP for the B site in the complete complex is much greater than the affinity of for the A site in the complete complex, or the affinity of a single RTP monomer to the B site alone. According to the model, only the affinity of the complex RTP for the ''Ter''-B (K3 in Figure X) is sufficient to prevent the removal of RTP from DNA when the replication fork moves along the DNA; therefore, if the replisome approaches from the B site, the RTP is not removed from the DNA and the replication fork is arrested. Similarly, when the replication fork approaches from the A site the binding affinity (K4 in Figure X) is not sufficient to prevent the removal of RTP and the replisome is able to pass.<ref>Kralicek, AV, Wilson PK, Ralston GB, Wake RG, King GF (1997) Reorganization of terminator DNA upon binding replication terminator protein: implications for the functional replication fork arrest complex. ''Nucleic Acids Research'' 25(3): 590-596.</ref> | ||
| Line 86: | Line 46: | ||
This mutational data provided by Duggin ''et al.'' (2004) suggested that replication fork arrest was a more complex process than one based purely on binding between RTP and DNA. In 2009, Duggin proposed the alternate theory that the C-terminus of an RTP monomer is able to contact the oncoming helicase and that this interaction is responsible for replication fork arrest. To test this hypothesis, Duggin created a series of RTP fusion proteins which were constructed by adding a GFP peptide to the C-terminus using a variety of spacer amino acids. Because the C-terminus is not in contact with DNA, the fusion proteins had no affect on the DNA binding properties of RTP; however, the fusion proteins had significantly reduced fork arrest efficiencies. These results effectively rule out the molecular clamp model, showing that RTP activity is independent of DNA binding affinity and suggesting that this C-terminal interaction with helicase is responsible for the fork arrest properties of RTP.<ref>Duggin, IG (2006) DNA Replication Fork Arrest by the ''Bacillus subtilis'' RTP-DNA complex involves a mechanism that is independent of the affinity of RTP-DNA binding. ''Journal of Molecular Biology'' 361: 1-6.</ref> | This mutational data provided by Duggin ''et al.'' (2004) suggested that replication fork arrest was a more complex process than one based purely on binding between RTP and DNA. In 2009, Duggin proposed the alternate theory that the C-terminus of an RTP monomer is able to contact the oncoming helicase and that this interaction is responsible for replication fork arrest. To test this hypothesis, Duggin created a series of RTP fusion proteins which were constructed by adding a GFP peptide to the C-terminus using a variety of spacer amino acids. Because the C-terminus is not in contact with DNA, the fusion proteins had no affect on the DNA binding properties of RTP; however, the fusion proteins had significantly reduced fork arrest efficiencies. These results effectively rule out the molecular clamp model, showing that RTP activity is independent of DNA binding affinity and suggesting that this C-terminal interaction with helicase is responsible for the fork arrest properties of RTP.<ref>Duggin, IG (2006) DNA Replication Fork Arrest by the ''Bacillus subtilis'' RTP-DNA complex involves a mechanism that is independent of the affinity of RTP-DNA binding. ''Journal of Molecular Biology'' 361: 1-6.</ref> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | <Structure load='2I06' size='400' frame='true' align='right' caption='Locked Tus-''Ter'' Complex (Mulcair ''et al.'' 2006)' scene='RTP_and_Tus/Co-ordination_of_his144/9' /> | ||
== Tus: An asymmetric monomer and unlikely candidate. == | == Tus: An asymmetric monomer and unlikely candidate. == | ||
| - | + | <scene name='RTP_and_Tus/Co-ordination_of_his144/9'>Locked Tus-Ter Complex</scene> (Mulcair ''et al.'' 2006, [[2l06]]) | |
| Line 115: | Line 70: | ||
A stepwise model of dissociation of Tus from ''Ter'' DNA appealed to Neylon ''et al''. This would involve the formation of a nonspecific Tus-DNA complex before the formation of a specific Tus-''Ter'' complex during binding. According to this model, as DnaB approaches from the permissive end it promotes the formation of the lower-affinity nonspecific complex, which would then rapidly dissociate; conversely, when DnaB approaches from the nonpermissive face, formation of the nonspecific complex would be prevented and Tus would become kinetically locked onto the ''Ter'' DNA.<ref>Neylon C, Kralicek AV, Hill TM, Dixon NE (2005) Replication Termination in Escherichia coli: Structure and Antihelicase Activity of the Tus-Ter Complex. ''Microbiology and Molecular Biology Reviews'' 69: 501 - 526. </ref> | A stepwise model of dissociation of Tus from ''Ter'' DNA appealed to Neylon ''et al''. This would involve the formation of a nonspecific Tus-DNA complex before the formation of a specific Tus-''Ter'' complex during binding. According to this model, as DnaB approaches from the permissive end it promotes the formation of the lower-affinity nonspecific complex, which would then rapidly dissociate; conversely, when DnaB approaches from the nonpermissive face, formation of the nonspecific complex would be prevented and Tus would become kinetically locked onto the ''Ter'' DNA.<ref>Neylon C, Kralicek AV, Hill TM, Dixon NE (2005) Replication Termination in Escherichia coli: Structure and Antihelicase Activity of the Tus-Ter Complex. ''Microbiology and Molecular Biology Reviews'' 69: 501 - 526. </ref> | ||
| - | |||
'''So how does Tus stop the replication fork, and why is it a polar arrest mechanism?''' | '''So how does Tus stop the replication fork, and why is it a polar arrest mechanism?''' | ||
| Line 124: | Line 78: | ||
The locked Tus-''Ter'' complex is the most stable known monomeric DNA binding protein with a double-stranded sequence-specific recognition sequence, with a reported half life of 550min (Mulcair ''et al.'', 2006). The formation of a large hydrogen-bond network is critical to sequence recognition and the stability of the twisted β-strands lying across the major groove.<ref>Mulcair MD, Schaeffer PM, Oakley AJ, Cross HF, Neylon C, Hill TM, Dixon NE (2006) A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. ''Cell'' 125: 1309 - 1319.</ref> | The locked Tus-''Ter'' complex is the most stable known monomeric DNA binding protein with a double-stranded sequence-specific recognition sequence, with a reported half life of 550min (Mulcair ''et al.'', 2006). The formation of a large hydrogen-bond network is critical to sequence recognition and the stability of the twisted β-strands lying across the major groove.<ref>Mulcair MD, Schaeffer PM, Oakley AJ, Cross HF, Neylon C, Hill TM, Dixon NE (2006) A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. ''Cell'' 125: 1309 - 1319.</ref> | ||
| - | |||
| - | |||
'''Just when we thought we had a nice, elegant theory...''' | '''Just when we thought we had a nice, elegant theory...''' | ||
In 2008, Bastia ''et al.'' proposed that Tus is actually a polar antitranslocase, and that an AT-GC transversion at position 6 did not affect DnaB-translocation ''in vitro''. They suggested that the base-flipping of C6 functions as a fail-safe mechanism, and that the replication fork is halted primarily by Tus-DnaB and Tus-''Ter'' interactions.<ref>Bastia D, Zzaman S, Krings G, Saxena M, Peng XH, Greenberg MM (2008) Replication termination mechanism as revealed by Tus-mediated polar arrest of a sliding helicase. ''Proceedings of the National Academy of Sciences'' 105: 12831 - 12836.</ref> | In 2008, Bastia ''et al.'' proposed that Tus is actually a polar antitranslocase, and that an AT-GC transversion at position 6 did not affect DnaB-translocation ''in vitro''. They suggested that the base-flipping of C6 functions as a fail-safe mechanism, and that the replication fork is halted primarily by Tus-DnaB and Tus-''Ter'' interactions.<ref>Bastia D, Zzaman S, Krings G, Saxena M, Peng XH, Greenberg MM (2008) Replication termination mechanism as revealed by Tus-mediated polar arrest of a sliding helicase. ''Proceedings of the National Academy of Sciences'' 105: 12831 - 12836.</ref> | ||
| - | |||
| - | |||
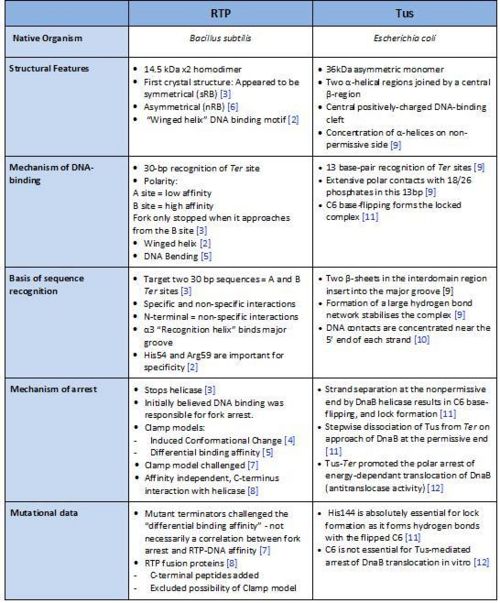
== Comparison of RTP and Tus == | == Comparison of RTP and Tus == | ||
[[Image:Comparison of RTP and Tus.jpg|500px|centre|thumb| A summary of the different features of fork arrest proteins RTP and Tus ]] | [[Image:Comparison of RTP and Tus.jpg|500px|centre|thumb| A summary of the different features of fork arrest proteins RTP and Tus ]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
== Replication Fork Termination: The Future of Discoveries == | == Replication Fork Termination: The Future of Discoveries == | ||
| Line 146: | Line 91: | ||
Perhaps surprisingly, replication fork termination has also been identified in specific regions of eukaryotes chromosoms. In the yeast ''Saccharomyces cerevisiae'' the terminator protein Fob1p has been shown to arrest the replication fork at ''Ter'' sites in non-transcribed spacer regions of rDNA; while in ''Schizosaccharomyes pombe'' polar replication termination by RTS1 has been shown to control the direction of replication of the ''mat1'' mating locus, enabling the yeast to alternate between mating types. This provides evidence that the replication termination process has been adapted by a wide range of organisms and is able to perform a variety of functions, opening up an exciting new field of research.<ref>Kaplan DL, Bastia D (2009) Mechanisms of polar arrest of a replication fork. ''Molecular Microbiology'' 72(2): 279-285.</ref> | Perhaps surprisingly, replication fork termination has also been identified in specific regions of eukaryotes chromosoms. In the yeast ''Saccharomyces cerevisiae'' the terminator protein Fob1p has been shown to arrest the replication fork at ''Ter'' sites in non-transcribed spacer regions of rDNA; while in ''Schizosaccharomyes pombe'' polar replication termination by RTS1 has been shown to control the direction of replication of the ''mat1'' mating locus, enabling the yeast to alternate between mating types. This provides evidence that the replication termination process has been adapted by a wide range of organisms and is able to perform a variety of functions, opening up an exciting new field of research.<ref>Kaplan DL, Bastia D (2009) Mechanisms of polar arrest of a replication fork. ''Molecular Microbiology'' 72(2): 279-285.</ref> | ||
| + | </StructureSection> | ||
| + | ==3D structures of replication terminator protein== | ||
| - | + | [[Replication Termination Protein]] | |
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
3D structures of replication terminator protein
Replication Termination Protein
References
- ↑ Wake, RG and King, GF (1997) A tale of two terminators: crystal structures sharpen the debate on DNA replication fork arrest mechanisms. Structure 5: 1-5.
- ↑ Wilce JA, Vivian JP, Hastings AF, Otting G, Folmer RHA, Duggin IG, Wake RG, Wilce MCJ (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. Nature Structural Biology 8: 206-210.
- ↑ Bussiere DE, Bastia D, White SW (1995) Crystal structure of the replication terminator protein from B. subtilis at 2.6 A. Cell 80(4): 651-60.
- ↑ Smith MT, de Vries CJ, Langley DB, King GF, Wake RG (1996) The Bacillis subtilis DNA Replication Terminator. Journal of Molecular Biology 260: 54-69.
- ↑ Kralicek, AV, Wilson PK, Ralston GB, Wake RG, King GF (1997) Reorganization of terminator DNA upon binding replication terminator protein: implications for the functional replication fork arrest complex. Nucleic Acids Research 25(3): 590-596.
- ↑ Vivian JP, Porter CJ, Wilce JA, Wilce MCJ (2007) An asymmetric structure of the Bacillus subtilis Replication Terminator Protein in complex with DNA. Journal of Molecular Biology 370: 481-491.
- ↑ Duggin IG, Matthews JM, Dixon, NE, Wake RG, Mackay JP (2004) A Complex Mechanism Determines Polarity of DNA Replication Fork Arrest by the Replication Terminator Complex of Bacillus subtilis. The Journal of Biological Chemistry 280(13): 13105-13113.
- ↑ Duggin, IG (2006) DNA Replication Fork Arrest by the Bacillus subtilis RTP-DNA complex involves a mechanism that is independent of the affinity of RTP-DNA binding. Journal of Molecular Biology 361: 1-6.
- ↑ Kamada K, Horiuchi T, Ohsumi K, Shimamoto N, Morikawa K (1996) Structure of a replication-terminator protein complexed with DNA. Nature 383: 598 - 603.
- ↑ Neylon C, Kralicek AV, Hill TM, Dixon NE (2005) Replication Termination in Escherichia coli: Structure and Antihelicase Activity of the Tus-Ter Complex. Microbiology and Molecular Biology Reviews 69: 501 - 526.
- ↑ Mulcair MD, Schaeffer PM, Oakley AJ, Cross HF, Neylon C, Hill TM, Dixon NE (2006) A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. Cell 125: 1309 - 1319.
- ↑ Bastia D, Zzaman S, Krings G, Saxena M, Peng XH, Greenberg MM (2008) Replication termination mechanism as revealed by Tus-mediated polar arrest of a sliding helicase. Proceedings of the National Academy of Sciences 105: 12831 - 12836.
- ↑ Kaplan DL, Bastia D (2009) Mechanisms of polar arrest of a replication fork. Molecular Microbiology 72(2): 279-285.