Group:MUZIC:Myopodin
From Proteopedia

| (75 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | ===Synaptopodin-2=== | |
| - | == | + | ==Introduction== |
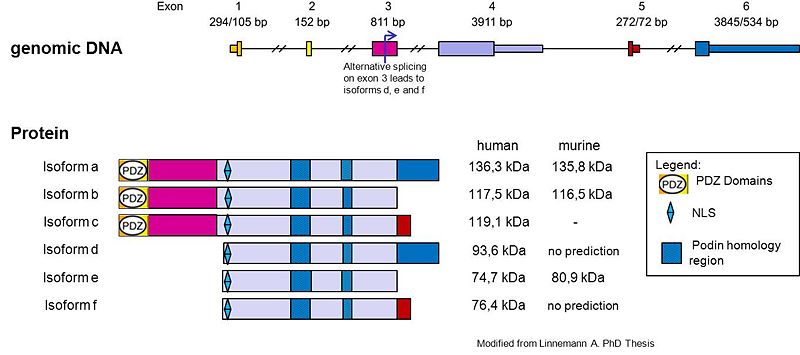
| - | + | The protein synaptopodin-2 (encoded by the gene SYNPO2[http://www.uniprot.org/uniprot/Q9UMS6]), also called '''myopodin''', genethonin-2, synaptopodin-like and fesselin, is together with synaptopodin and tritopodin/CHAP a member of the podin protein family. It is widely expressed in striated, heart and smooth muscle cells where it localizes to Z-disks and dense bodies, respectively <ref name="r1"> PMID:11673475 </ref>. Myopodin is a multiadapter protein that interacts with filamentous actin, α-actinin and filamin. Different isoforms result from alternative splicing and alternative promoter usage and the predicted size of the resulting proteins varies between 74 kDa and 136 kDa; these variants show differential tissue distribution. The discrepancy between calculated molecular mass and apparent mobility in SDS-PAGE is at present still unclear, but might be due to post-translational modifications <ref> PMID:20554076 </ref> <ref> PMID:18371299 </ref> <ref> PMID:11696420</ref> <ref> PMID:12917631 </ref> <ref> PMID:18588515 </ref>. The lower molecular mass myopodin isoforms are dual compartment proteins that redistribute between the nucleus and the cytoplasm in a differentiation-dependent and stress-induced manner <ref> PMID:C2150840 </ref>. Although its biological function is still undefined, recent findings support a role for myopodin as a z-disc multiadaptor protein (96). | |
| - | + | ||
| - | + | ||
| + | ==Sequence Annotation== | ||
| + | Myopodin contains one PPXY motif and multiple PXXP motifs <ref name="r1"> PMID:11673475 </ref>. Beside 2 lysine-rich NLS sites (consensus motif K-K/RX-K/R) myopodin provides 2 binding sites for 14-3-3(consensus motif a: RSXpS/TXP; b: RXXXpS/TXP Biophys Rev (2010) 2:181-189). These sites seem to be indispensable for effective shuttling of myopodin from the Z-disk into the nucleus <ref> PMCID:PMC2171942 </ref>. Phosphorylation of myopodin within the 14-3-3 binding sites (S225 and T272) by protein kinase A and Ca<sup>2+</sup>/calmodulin-dependent protein kinase II abrogates the binding with α -actinin and promotes the binding with 14-3-3 and importin α <ref> PMID:17923693 </ref>. Another important domain present in myopodin is the PDZ domain (postsynaptic density 95, discs large, and zonula occludens-1). PDZ domains are protein-protein interaction modules that can mediate multiple biological processes such as vesicle transport, ion channel signaling, and signal transduction in several tissues. PDZ domains in myopodin are of unknown function <ref> PMID:18371299 </ref>. | ||
| - | [[Image:Myopodin.jpg|700px]] | ||
| + | [[Image:Myopodin.jpg|800px]] | ||
| + | |||
| + | |||
| + | ==Function== | ||
| + | Myopodin is a dual compartment protein that redistributes between the nucleus and the cytoplasm in a differentiation-dependent and stress-induced fashion, and in addition displays actin-bundling activity <ref name="r1"> PMID:11673475 </ref>. | ||
| + | In undifferentiated myoblasts, myopodin seems to be expressed preferentially in the nucleus and only weakly in the cytoplasm, whereas in differentiating myotubes, myopodin is incorporated into the Z-disc with no detectable nuclear expression, suggesting that myopodin may be involved in the regulation of myocyte differentiation <ref name="r1"> PMID:11673475 </ref> <ref> PMID:19360353 </ref>. | ||
| + | Myopodin has also been identified as a tumor suppressor gene that is frequently deleted in aggressive prostate cancer <ref> PMID:15111326 </ref>. Expression of myopodin protein suppresses both tumor growth and metastasis in vitro and in vivo <ref> PMID:15111326 </ref>. | ||
== Myopodin Interactions == | == Myopodin Interactions == | ||
| - | Actin was the first binding partner of | + | Actin was the first binding partner of myopodin to be identified. Actin binding was narrowed down to residues 410–563 of murine myopodin <ref name="r1"> PMID:11673475 </ref>. |
| - | Myopodin binds to α-actinin | + | Myopodin also binds to α-actinin via its spectrin repeats <ref> PMID:16450054 </ref>, <ref> PMID:20554076 </ref> . Myopodin may be generally involved in the organization and anchoring of actin in muscle cells and might be required for the correct localization of α-actinin. This hypothesis is supported by recent findings where myopodin expression precedes actin and α-actinin expression <ref> PMID:20554076 </ref>. |
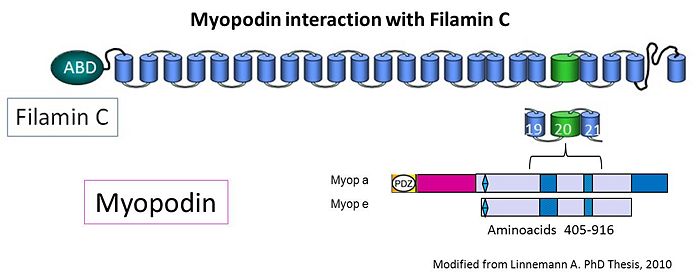
| - | A | + | A filamin-binding region was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin <ref> PMID:20554076 </ref>. |
| - | + | Yeast two-hybrid analysis also identified an interaction of myopodin with zyxin, which leads to slower migration of prostate cancer cells and reduced invasiveness <ref> PMID:16885336 </ref>. | |
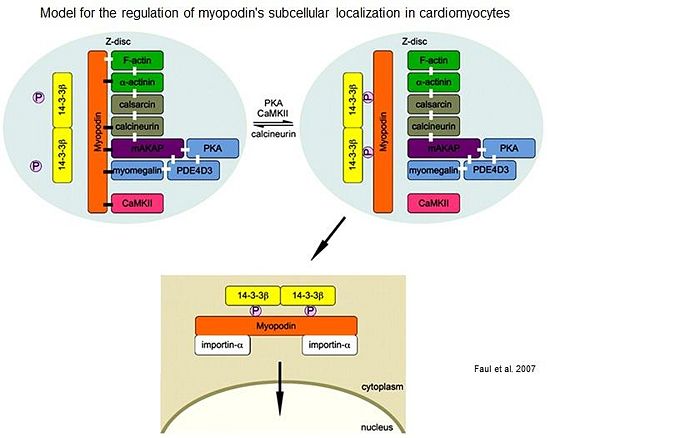
| + | In cardiomyocytes, myopodin forms a Z-disc signaling complex with α-actinin, calcineurin, Ca<sup>2+</sup>/calmodulin-dependent kinase II (CaMKII), muscle-specific A-kinase anchoring protein, and myomegalin. Calcineurin keeps myopodin dephosphorylated preventing 14-3-3ß binding to myopodin. Upon activation of PKA/CaMKII or inhibition of calcineurin, myopodin undergoes phosphorylation, enabling its interaction with 14-3-3ß, which releases myopodin from the Z-disc-anchoring proteins and induces its nuclear import. This novel intracellular signaling pathway suggests that changes in Z-disc dynamics may translate into compartmentalized signal transduction in the heart <ref> PMID:17923693 </ref>. | ||
| + | |||
| + | [[Image:Myopodin interaction with Filamin C.jpg|700px]] | ||
| + | [[Image:Model for the regulation of Myopodin's subcellular localization in Cardiomyocytes.jpg|700px]] | ||
| - | + | ==Pathology== | |
| + | The SYNPO2 gene frequently exhibits deletions in aggresive prostate cancer tissues and statistical analysis indicates that deletion of myopodin is highly correlated with the invasiveness of prostate cancers, and thus may hold promise as an important prognostic marker for prostate cancers <ref> PMID:11696420</ref>. Myopodin was suggested to play a role as tumor suppressor, because in normal urothelium of the bladder myopodin is localized both in the cytoplasm and in the nucleus, wheras in superficial and invasive bladder tumors, reduced nuclear expression of myopodin was observed <ref> PMID:12917631 </ref>. The expression of myopodin suppressed tumor growth and metastasis <ref> PMID:15111326 </ref>. | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | [[Category: Z-disk]] | ||
Current revision
Contents |
Synaptopodin-2
Introduction
The protein synaptopodin-2 (encoded by the gene SYNPO2[1]), also called myopodin, genethonin-2, synaptopodin-like and fesselin, is together with synaptopodin and tritopodin/CHAP a member of the podin protein family. It is widely expressed in striated, heart and smooth muscle cells where it localizes to Z-disks and dense bodies, respectively [1]. Myopodin is a multiadapter protein that interacts with filamentous actin, α-actinin and filamin. Different isoforms result from alternative splicing and alternative promoter usage and the predicted size of the resulting proteins varies between 74 kDa and 136 kDa; these variants show differential tissue distribution. The discrepancy between calculated molecular mass and apparent mobility in SDS-PAGE is at present still unclear, but might be due to post-translational modifications [2] [3] [4] [5] [6]. The lower molecular mass myopodin isoforms are dual compartment proteins that redistribute between the nucleus and the cytoplasm in a differentiation-dependent and stress-induced manner [7]. Although its biological function is still undefined, recent findings support a role for myopodin as a z-disc multiadaptor protein (96).
Sequence Annotation
Myopodin contains one PPXY motif and multiple PXXP motifs [1]. Beside 2 lysine-rich NLS sites (consensus motif K-K/RX-K/R) myopodin provides 2 binding sites for 14-3-3(consensus motif a: RSXpS/TXP; b: RXXXpS/TXP Biophys Rev (2010) 2:181-189). These sites seem to be indispensable for effective shuttling of myopodin from the Z-disk into the nucleus [8]. Phosphorylation of myopodin within the 14-3-3 binding sites (S225 and T272) by protein kinase A and Ca2+/calmodulin-dependent protein kinase II abrogates the binding with α -actinin and promotes the binding with 14-3-3 and importin α [9]. Another important domain present in myopodin is the PDZ domain (postsynaptic density 95, discs large, and zonula occludens-1). PDZ domains are protein-protein interaction modules that can mediate multiple biological processes such as vesicle transport, ion channel signaling, and signal transduction in several tissues. PDZ domains in myopodin are of unknown function [10].
Function
Myopodin is a dual compartment protein that redistributes between the nucleus and the cytoplasm in a differentiation-dependent and stress-induced fashion, and in addition displays actin-bundling activity [1]. In undifferentiated myoblasts, myopodin seems to be expressed preferentially in the nucleus and only weakly in the cytoplasm, whereas in differentiating myotubes, myopodin is incorporated into the Z-disc with no detectable nuclear expression, suggesting that myopodin may be involved in the regulation of myocyte differentiation [1] [11]. Myopodin has also been identified as a tumor suppressor gene that is frequently deleted in aggressive prostate cancer [12]. Expression of myopodin protein suppresses both tumor growth and metastasis in vitro and in vivo [13].
Myopodin Interactions
Actin was the first binding partner of myopodin to be identified. Actin binding was narrowed down to residues 410–563 of murine myopodin [1]. Myopodin also binds to α-actinin via its spectrin repeats [14], [15] . Myopodin may be generally involved in the organization and anchoring of actin in muscle cells and might be required for the correct localization of α-actinin. This hypothesis is supported by recent findings where myopodin expression precedes actin and α-actinin expression [16]. A filamin-binding region was mapped to a fragment encompassing amino acids 240–521 of myopodin, i.e. a region that contains two of the previously described homology regions shared by myopodin and synaptopodin [17]. Yeast two-hybrid analysis also identified an interaction of myopodin with zyxin, which leads to slower migration of prostate cancer cells and reduced invasiveness [18]. In cardiomyocytes, myopodin forms a Z-disc signaling complex with α-actinin, calcineurin, Ca2+/calmodulin-dependent kinase II (CaMKII), muscle-specific A-kinase anchoring protein, and myomegalin. Calcineurin keeps myopodin dephosphorylated preventing 14-3-3ß binding to myopodin. Upon activation of PKA/CaMKII or inhibition of calcineurin, myopodin undergoes phosphorylation, enabling its interaction with 14-3-3ß, which releases myopodin from the Z-disc-anchoring proteins and induces its nuclear import. This novel intracellular signaling pathway suggests that changes in Z-disc dynamics may translate into compartmentalized signal transduction in the heart [19].
Pathology
The SYNPO2 gene frequently exhibits deletions in aggresive prostate cancer tissues and statistical analysis indicates that deletion of myopodin is highly correlated with the invasiveness of prostate cancers, and thus may hold promise as an important prognostic marker for prostate cancers [20]. Myopodin was suggested to play a role as tumor suppressor, because in normal urothelium of the bladder myopodin is localized both in the cytoplasm and in the nucleus, wheras in superficial and invasive bladder tumors, reduced nuclear expression of myopodin was observed [21]. The expression of myopodin suppressed tumor growth and metastasis [22].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J Cell Biol. 2001 Oct 29;155(3):393-404. Epub 2001 Oct 22. PMID:11673475 doi:10.1083/jcb.200012039
- ↑ Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Furst DO. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010 Sep;89(9):681-92. Epub 2010 May 31. PMID:20554076 doi:10.1016/j.ejcb.2010.04.004
- ↑ De Ganck A, De Corte V, Staes A, Gevaert K, Vandekerckhove J, Gettemans J. Multiple isoforms of the tumor suppressor myopodin are simultaneously transcribed in cancer cells. Biochem Biophys Res Commun. 2008 May 30;370(2):269-73. Epub 2008 Mar 25. PMID:18371299 doi:10.1016/j.bbrc.2008.03.086
- ↑ Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P, Dhir R, Krill D, Becich MJ, Michalopoulos G, Finkelstein S, Luo JH. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am J Pathol. 2001 Nov;159(5):1603-12. PMID:11696420 doi:10.1016/S0002-9440(10)63006-4
- ↑ Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P. Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene. 2003 Aug 14;22(34):5298-305. PMID:12917631 doi:http://dx.doi.org/10.1038/sj.onc.1206616
- ↑ Schroeter MM, Beall B, Heid HW, Chalovich JM. In vitro characterization of native mammalian smooth-muscle protein synaptopodin 2. Biosci Rep. 2008 Aug;28(4):195-203. PMID:18588515 doi:10.1042/BSR20080079
- ↑ PMID:C2150840
- ↑ PMCID:PMC2171942
- ↑ Faul C, Dhume A, Schecter AD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol Cell Biol. 2007 Dec;27(23):8215-27. Epub 2007 Oct 8. PMID:17923693 doi:10.1128/MCB.00950-07
- ↑ De Ganck A, De Corte V, Staes A, Gevaert K, Vandekerckhove J, Gettemans J. Multiple isoforms of the tumor suppressor myopodin are simultaneously transcribed in cancer cells. Biochem Biophys Res Commun. 2008 May 30;370(2):269-73. Epub 2008 Mar 25. PMID:18371299 doi:10.1016/j.bbrc.2008.03.086
- ↑ De Ganck A, De Corte V, Bruyneel E, Bracke M, Vandekerckhove J, Gettemans J. Down-regulation of myopodin expression reduces invasion and motility of PC-3 prostate cancer cells. Int J Oncol. 2009 May;34(5):1403-9. PMID:19360353
- ↑ Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D, Cieply K, Wells A, Luo JH. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004 May;164(5):1799-806. PMID:15111326 doi:10.1016/S0002-9440(10)63738-8
- ↑ Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D, Cieply K, Wells A, Luo JH. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004 May;164(5):1799-806. PMID:15111326 doi:10.1016/S0002-9440(10)63738-8
- ↑ Pham M, Chalovich JM. Smooth muscle alpha-actinin binds tightly to fesselin and attenuates its activity toward actin polymerization. J Muscle Res Cell Motil. 2006;27(1):45-51. Epub 2006 Feb 1. PMID:16450054 doi:10.1007/s10974-005-9053-2
- ↑ Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Furst DO. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010 Sep;89(9):681-92. Epub 2010 May 31. PMID:20554076 doi:10.1016/j.ejcb.2010.04.004
- ↑ Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Furst DO. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010 Sep;89(9):681-92. Epub 2010 May 31. PMID:20554076 doi:10.1016/j.ejcb.2010.04.004
- ↑ Linnemann A, van der Ven PF, Vakeel P, Albinus B, Simonis D, Bendas G, Schenk JA, Micheel B, Kley RA, Furst DO. The sarcomeric Z-disc component myopodin is a multiadapter protein that interacts with filamin and alpha-actinin. Eur J Cell Biol. 2010 Sep;89(9):681-92. Epub 2010 May 31. PMID:20554076 doi:10.1016/j.ejcb.2010.04.004
- ↑ Yu YP, Luo JH. Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Res. 2006 Aug 1;66(15):7414-9. PMID:16885336 doi:10.1158/0008-5472.CAN-06-0227
- ↑ Faul C, Dhume A, Schecter AD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol Cell Biol. 2007 Dec;27(23):8215-27. Epub 2007 Oct 8. PMID:17923693 doi:10.1128/MCB.00950-07
- ↑ Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P, Dhir R, Krill D, Becich MJ, Michalopoulos G, Finkelstein S, Luo JH. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am J Pathol. 2001 Nov;159(5):1603-12. PMID:11696420 doi:10.1016/S0002-9440(10)63006-4
- ↑ Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P. Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene. 2003 Aug 14;22(34):5298-305. PMID:12917631 doi:http://dx.doi.org/10.1038/sj.onc.1206616
- ↑ Jing L, Liu L, Yu YP, Dhir R, Acquafondada M, Landsittel D, Cieply K, Wells A, Luo JH. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004 May;164(5):1799-806. PMID:15111326 doi:10.1016/S0002-9440(10)63738-8