We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Pyruvate Kinase
From Proteopedia
(Difference between revisions)
| (29 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
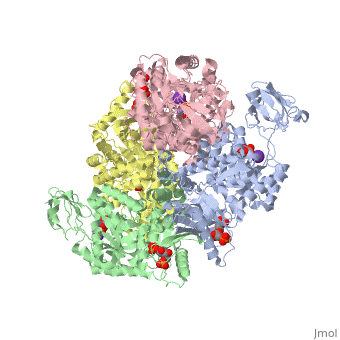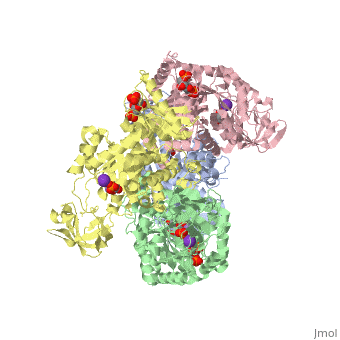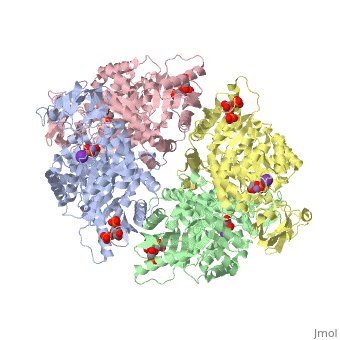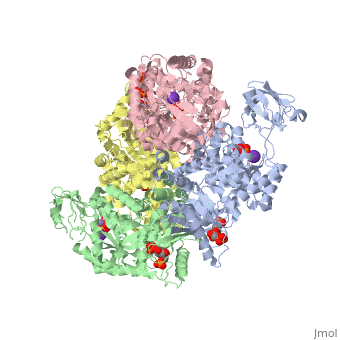
| - | == | + | <StructureSection load='2vgb' size='350' side='right' scene='' caption='Human pyruvate kinase tetramer complex with fructose diphosphate, phosphoglycolic acid, Mn+2 and K+ (purple) ions (PDB code [[2vgb]])'> |
| - | [[Pyruvate Kinase]] is an enzyme that is involved in glycolysis. Pyruvate kinase’s function is to catalyze the last step of glycolysis; thereby, generating the second ATP of glycolysis and pyruvate. It is able to catalyze this step by transferring the phosphate group from phosphoenolpyruvate (PEP) to ADP <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=501|}}</ref>. | + | [[Pyruvate Kinase]] is an enzyme that is involved in glycolysis. Pyruvate kinase’s function is to catalyze the last step of glycolysis; thereby, generating the second ATP of glycolysis and pyruvate. It is able to catalyze this step by transferring the phosphate group from phosphoenolpyruvate (PEP) to ADP <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=501|}}</ref>. See [[Glycolysis Enzymes]]. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Structure== | ==Structure== | ||
This particular protein is found in Homo sapiens and has the abbreviation PK. Pyruvate kinase belongs to the all beta proteins class and has the PK beta-barrel domain-like fold. It belongs to the PK beta-barrel domain-like superfamily and pyruvate kinase beta-barrel domain family<ref>{{website| title=SCOP: Protein: Pyruvate kinase (PK) from Human (Homo sapiens) [TaxId: 9606]|url=http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html|}}</ref>. | This particular protein is found in Homo sapiens and has the abbreviation PK. Pyruvate kinase belongs to the all beta proteins class and has the PK beta-barrel domain-like fold. It belongs to the PK beta-barrel domain-like superfamily and pyruvate kinase beta-barrel domain family<ref>{{website| title=SCOP: Protein: Pyruvate kinase (PK) from Human (Homo sapiens) [TaxId: 9606]|url=http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html|}}</ref>. | ||
| - | Though pyruvate kinase is classified into all beta proteins, pyruvate kinase's <scene name='Keegan_Gelvoria_Sandbox_1/Secondary_structure/1'>secondary structure</scene> comprises of both alpha helices and beta sheets. In the quaternary structure of pyruvate kinase, it can be observed to have <scene name='Keegan_Gelvoria_Sandbox_1/Structure_4_domains/1'>four domains</scene> in humans. Thus, this enzyme is tetrameric with <scene name='Keegan_Gelvoria_Sandbox_1/Metal_binding_sites/1'>metal binding sites</scene> on each domain for the <scene name='Keegan_Gelvoria_Sandbox_1/Ligands/1'>K+</scene> and Mg2+ ligands to bind to. There are four types of tissue-specific isozymes: L (liver), R (red blood cells), M1 (muscle, heart, and brain), and M2 (early fetal tissue)<ref>{{website| title=SCOP: Protein: Pyruvate kinase (PK) from Human (Homo sapiens) [TaxId: 9606]|url=http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html|}}</ref>. | + | Though pyruvate kinase is classified into all beta proteins, pyruvate kinase's <scene name='Keegan_Gelvoria_Sandbox_1/Secondary_structure/1'>secondary structure</scene> comprises of both alpha helices and beta sheets. In the quaternary structure of pyruvate kinase, it can be observed to have <scene name='Keegan_Gelvoria_Sandbox_1/Structure_4_domains/1'>four domains</scene> in humans. Thus, this enzyme is tetrameric with <scene name='Keegan_Gelvoria_Sandbox_1/Metal_binding_sites/1'>metal binding sites</scene> on each domain for the <scene name='Keegan_Gelvoria_Sandbox_1/Ligands/1'>K+</scene> and Mg2+ ligands to bind to. There are four types of tissue-specific isozymes: '''L''' (liver), '''R''' (red blood cells), '''M1''' (muscle, heart, and brain), and '''M2''' (early fetal tissue)<ref>{{website| title=SCOP: Protein: Pyruvate kinase (PK) from Human (Homo sapiens) [TaxId: 9606]|url=http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html|}}</ref>. |
==Mechanism== | ==Mechanism== | ||
| - | + | ||
| - | + | ||
<scene name='Keegan_Gelvoria_Sandbox_1/N_c_rainbow/null'>Pyruvate Kinase</scene> catalyzes the final reaction of glycolysis. It couples the free energy of PEP cleavage to the generation of ATP during the synthesis of the final product, pyruvate. This reaction necessitates one K+ and two Mg2+ cations to be used in two steps. The first step is the nucleophilic attack of the PEP phosphorous atom by β-phosphoryl oxygen of ADP; this step displaces enolpyruvate while forming ATP. In the second step, enolpyruvate tautomerizes to pyruvate <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=502|}}</ref>. The formation of a high-energy intermediate by enolase in the 9th reaction of glycolysis allows for the synthesis of ATP in this reaction. Though the hydrolysis of 2PG is insufficient in driving the synthesis of ATP, the dehydration of 2PG allows for such a reaction to occur by forming a high-energy intermediate. The high potential of PEP reflects the large release of energy that occurs with the conversion of enolpyruvate to its keto tautomer, pyruvate <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=503|}}</ref>. | <scene name='Keegan_Gelvoria_Sandbox_1/N_c_rainbow/null'>Pyruvate Kinase</scene> catalyzes the final reaction of glycolysis. It couples the free energy of PEP cleavage to the generation of ATP during the synthesis of the final product, pyruvate. This reaction necessitates one K+ and two Mg2+ cations to be used in two steps. The first step is the nucleophilic attack of the PEP phosphorous atom by β-phosphoryl oxygen of ADP; this step displaces enolpyruvate while forming ATP. In the second step, enolpyruvate tautomerizes to pyruvate <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=502|}}</ref>. The formation of a high-energy intermediate by enolase in the 9th reaction of glycolysis allows for the synthesis of ATP in this reaction. Though the hydrolysis of 2PG is insufficient in driving the synthesis of ATP, the dehydration of 2PG allows for such a reaction to occur by forming a high-energy intermediate. The high potential of PEP reflects the large release of energy that occurs with the conversion of enolpyruvate to its keto tautomer, pyruvate <ref>{{book |author=Voet, Donald; Voet, Judith C.; Pratt, Charlotte W.|title=Fundamentals of Biochemistry: Life at the Molecular Level|edition= 3|pages=503|}}</ref>. | ||
| Line 20: | Line 14: | ||
In the glycolytic cycle, there are three compounds that have a large negative ∆G which includes the reaction pyruvate kinase catalyzes. Due to these three steps regulating the overall activity of the cycle, they are generally irreversible in vivo. Through numerous studies, the activity of pyruvate kinase has been found to be regulated by these effectors. | In the glycolytic cycle, there are three compounds that have a large negative ∆G which includes the reaction pyruvate kinase catalyzes. Due to these three steps regulating the overall activity of the cycle, they are generally irreversible in vivo. Through numerous studies, the activity of pyruvate kinase has been found to be regulated by these effectors. | ||
| - | |||
a. Phosphoenolpyruvate, the substrate, can impact enzymatic activity by enhancing the reaction | a. Phosphoenolpyruvate, the substrate, can impact enzymatic activity by enhancing the reaction | ||
| Line 28: | Line 21: | ||
c. Alanine has also been found to be a negative allosteric modulator <ref>PMID:629752 </ref>. | c. Alanine has also been found to be a negative allosteric modulator <ref>PMID:629752 </ref>. | ||
| - | |||
This reaction, although appearing reversible, is essentially irreversible under physiological conditions, thus helping control the metabolic flux in glycolysis. Through allosteric regulation, the PEP binding site is distorted by 29 degrees on transition from the R-state to the T-state. | This reaction, although appearing reversible, is essentially irreversible under physiological conditions, thus helping control the metabolic flux in glycolysis. Through allosteric regulation, the PEP binding site is distorted by 29 degrees on transition from the R-state to the T-state. | ||
| Line 36: | Line 28: | ||
The allosteric site is located 40 å from the active site and is entirely located in the enzyme regulatory (C) domain. A phosphate-binding site for the allosteric activator is created by residues encoded by a region of the gene that corresponds to spliced exons of mammalian isozymes <ref>PMID:9519410 </ref>. | The allosteric site is located 40 å from the active site and is entirely located in the enzyme regulatory (C) domain. A phosphate-binding site for the allosteric activator is created by residues encoded by a region of the gene that corresponds to spliced exons of mammalian isozymes <ref>PMID:9519410 </ref>. | ||
FBP activation induces several conformational changes among active-site sidechains through a mechanism that is most likely to involve significant domain motions. The conformational differences observed between the active sites of inactive and fully active Pyruvate Kinase enzymes is in agreement with the thermodynamic mechanism of allosteric activation through a metal relay that increases the affinity of the enzyme for its phosphoenolpyruvate substrate. | FBP activation induces several conformational changes among active-site sidechains through a mechanism that is most likely to involve significant domain motions. The conformational differences observed between the active sites of inactive and fully active Pyruvate Kinase enzymes is in agreement with the thermodynamic mechanism of allosteric activation through a metal relay that increases the affinity of the enzyme for its phosphoenolpyruvate substrate. | ||
| - | |||
Without a high K+ concentration, the kinetic mechanism of pyruvate kinase changes from random to ordered with phosphoenolpyruvate as the first substrate. Vmax with K+ was about 400 times higher than a wild type sample without K+. In the presence of K+, the affinities for phosphoenolpruvate and ADP were 2-6 times higher than in the abscence of K+. This shows that K+ is involved in the acquisition of the active conformation of the enzyme, allowing either phosphoenolpyruvate or ADP to bind independently, but without K+, ADP cannot bind to the enzyme until phosphoenolpyruvate forms a competent active site for an ordered mechanism. Wild type pyruvate kinase without K+ has an ordered rapid equilibrium kinetic mechanism that shows Vmax to be 0.8 +/- -.04 umol/min mg with a kcat of 3.2 s^-1. When the wild-type pyruvate kinase has K+, it is in a random rapid equilibrium kinetic mechanism with a Vmax of 299 +/- 11 umol/min mg with a kcat of 1182 <ref>PMID:16147999</ref>. | Without a high K+ concentration, the kinetic mechanism of pyruvate kinase changes from random to ordered with phosphoenolpyruvate as the first substrate. Vmax with K+ was about 400 times higher than a wild type sample without K+. In the presence of K+, the affinities for phosphoenolpruvate and ADP were 2-6 times higher than in the abscence of K+. This shows that K+ is involved in the acquisition of the active conformation of the enzyme, allowing either phosphoenolpyruvate or ADP to bind independently, but without K+, ADP cannot bind to the enzyme until phosphoenolpyruvate forms a competent active site for an ordered mechanism. Wild type pyruvate kinase without K+ has an ordered rapid equilibrium kinetic mechanism that shows Vmax to be 0.8 +/- -.04 umol/min mg with a kcat of 3.2 s^-1. When the wild-type pyruvate kinase has K+, it is in a random rapid equilibrium kinetic mechanism with a Vmax of 299 +/- 11 umol/min mg with a kcat of 1182 <ref>PMID:16147999</ref>. | ||
| Line 51: | Line 42: | ||
==3D structures of pyruvate kinase== | ==3D structures of pyruvate kinase== | ||
| + | [[Pyruvate kinase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Resources== | ==Additional Resources== | ||
| Line 107: | Line 52: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | [[Category: Topic Page]] | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008, 501-503.
- ↑ authors, The scop. "Structural Classification of Proteins". 2009. 2/26 2010. <http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html>.
- ↑ authors, The scop. "Structural Classification of Proteins". 2009. 2/26 2010. <http://scop.berkeley.edu/data/scop.b.c.jh.b.b.d.html>.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008, 501-503.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc., 2008, 501-503.
- ↑ Dann LG, Britton HG. Kinetics and mechanism of action of muscle pyruvate kinase. Biochem J. 1978 Jan 1;169(1):39-54. PMID:629752
- ↑ Mattevi A, Bolognesi M, Valentini G. The allosteric regulation of pyruvate kinase. FEBS Lett. 1996 Jun 24;389(1):15-9. PMID:8682196
- ↑ Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998 Feb 15;6(2):195-210. PMID:9519410
- ↑ Oria-Hernandez J, Cabrera N, Perez-Montfort R, Ramirez-Silva L. Pyruvate kinase revisited: the activating effect of K+. J Biol Chem. 2005 Nov 11;280(45):37924-9. Epub 2005 Sep 7. PMID:16147999 doi:10.1074/jbc.M508490200
- ↑ Dann LG, Britton HG. Kinetics and mechanism of action of muscle pyruvate kinase. Biochem J. 1978 Jan 1;169(1):39-54. PMID:629752
- ↑ Oria-Hernandez J, Cabrera N, Perez-Montfort R, Ramirez-Silva L. Pyruvate kinase revisited: the activating effect of K+. J Biol Chem. 2005 Nov 11;280(45):37924-9. Epub 2005 Sep 7. PMID:16147999 doi:10.1074/jbc.M508490200
- ↑ Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. Pyruvate kinase deficiency: the genotype-phenotype association. Blood Rev. 2007 Jul;21(4):217-31. Epub 2007 Mar 13. PMID:17360088 doi:10.1016/j.blre.2007.01.001
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Keegan Gelvoria, David Canner, Ann Taylor, Andrew Alexander