Sandbox Reserved 381
From Proteopedia
(/* Your Heading Here (maybe something like 'Structure')==<StructureSection load='1w3b' size='500' side='right' caption='Structure of HMG-CoA reductase (PDB entry 1w3b)' scene=''>Anything in this section will appear adjacent to the 3D structure an) |
|||
| (59 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | |
{{Sandbox_Reserved_JMeans}} | {{Sandbox_Reserved_JMeans}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | {{STRUCTURE_3pe4| PDB=3pe4 | SCENE= }} | ||
| + | <Structure load='3pe3' size='400' frame='true' align='right' caption='O-GlcNAc transferase with UDP' scene='Insert optional scene name here' /> | ||
| - | == ''O-GlcNAc transferase'' == | ||
| - | + | == O-GlcNAc transferase == | |
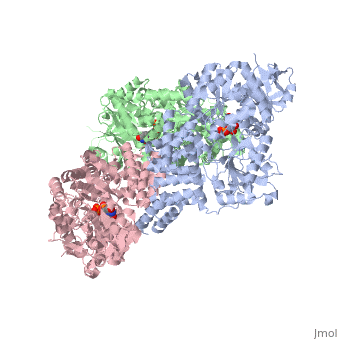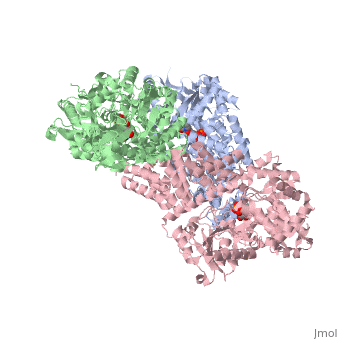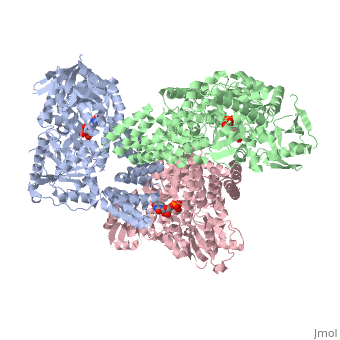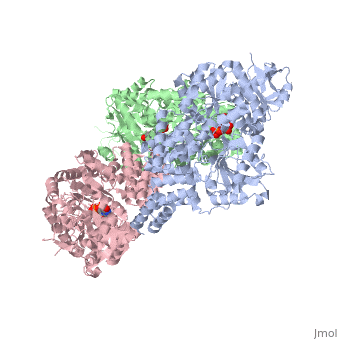
| + | O-linked β-N-acetylglucosamine transferase (O-GlcNAc transferase) is an essential mammalian enzyme that acts as a nutrient sensor, coupling metabolic status to the regulation of a wide variety of cellular signaling pathways.<ref> Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature.2007;446:1017-22.[http://www.nature.com/nature/journal/v446/n7139/abs/nature05815.html]</ref> OGT catalyses the transfer of N-acetylglucosamine from UDP-N-acetylglucosamine (UDP-GlcNAc) to serines and threonines of cytoplasmic, nuclear and mitochondrial proteins, including numerous transcription factors, tumour suppressors, kinases, phospahateses and histone-modifying proteins.<ref>PMID:21240259</ref> Two crystal structures of human OGT are reported here as a <scene name='Sandbox_Reserved_381/Ternary_complex_ogt_with_udp/1'>ternary complex</scene> with UDP and a <scene name='Sandbox_Reserved_381/Binary_complex_with_udp/1'>binary complex</scene> with UDP and a peptide substrate. | ||
| - | == O-GlcNAc | + | == O-GlcNAc transferase Function == |
| - | + | The major mechanism for nutrient sensing in eukaryotes involves OGT. OGT senses cellular glucose levels via UDP-GlcNAc concentration, and responds by O-GlcNAcylating a broad range of nuclear anf cytoplasmic proteins.<ref>PMID:17460662</ref> Insulin-like signaling pathways and transcriptional activators that regulate glucose levels by controlling gluconeogenisis include proteins that are O-GlcNAcylated by OGT.<ref>PMID:18288188</ref> Numerous O-GlcNAcylation sites are also phosphorylation sites. OGT is suggested to play a major role in modulating cellular kinase signaling cascades.<ref>PMID:12269319</ref> Widespread transcriptional regulations also involve OGT.<ref>PMID:19478141</ref> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | == OGT Modifications == | ||
| + | O-GlcNAc modification has been described for a large and still increasing number of proteins, many of which are key modulators of cellular signalling. O-GlcNAc modifications are catalysed by a OGT, and are removed by the antagonistic enzyme β-N-acetylglucosaminidase (O-GlcNAcase). The general scheme of O-linked N-acetylglucosamine modification suggests that N-acetylglucosamine is added to serine/threonine (Ser/Thr) residues of target proteins by the enzyme OGT, using UDP-GlcNAc as substrate. The N-acetylglucosamine group is removed by the antagonistic activity of O-GlcNAcase.<ref>Alexander G, Danilo G. The O-linked N-acetylglucosamine modification in cellular signalling and the immune system. EMBO reports. 2008 June;9:748-753[http://www.nature.com/embor/journal/v9/n8/full/embor2008129.html]</ref> | ||
| + | == OGT Features of Interest == | ||
| + | The OGT protein possess two ligands <scene name='Sandbox_Reserved_381/So4_ligand/1'>SO4</scene> and uridine-5-diphosphate <scene name='Sandbox_Reserved_381/Udp/1'>(UDP)</scene>. OGT is the only known member to glycosylate polypeptides, and it contains a long uncharacterized intervening sequence (~120 amino acids) in the middle of the catalytic region. Studies suggest that OGT contains a phosphatidylinositol (3,4,5)-trisphosphate (PIP<sub>3</sub>)binding domain. The most unusual feature of OGT is the intervening domain between the catalytic lobes, which is only found in metazoans. This polypeptide adopts a topologically novel fold with a seven-stranded <scene name='Sandbox_Reserved_381/Ogt_structure/1'>beta sheet</scene> core stabilized by flanking alpha helices. There are two long <scene name='Sandbox_Reserved_381/Unstructured_loops/1'>unstructured loops</scene> for which electron density is missing.<ref> PMID:18288188</ref> | ||
| + | == OGT Structure == | ||
| + | OGT is comprised of two distinct regions: a multidomain catalytic region, which has no available structure and an N-terminal region consisting of a series of tetratricopeptide repeat(TPR) units.<ref>PMID:9083067</ref> The N terminus of OGT is unusual, consisting of 2.5-13.5 tetratricopeptide repeats (TPRs) depending on alternative splicing.<ref>Kreppel L, Hart G. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015-32022</ref> The N-terminal domain of tetratricopeptide (TPR) mediates the recognition of a broad range of target proteins. Components of the nuclear pore complex are major OGT targets, as OGT depletion by RNA interference (RNAi) results in the loss of GlcNAc modification at the nuclear envelope. | ||
| + | == N Terminus == | ||
| + | <StructureSection load='1W3B' size='250' side='left' caption='Superhelical TPR domain of OGT, structural similarities to importin alpha. (PDB entry [[1W3B]])' scene=''>The crystal structure of the homodimeric TPR domain of human OGT, which contains 11.5 TPR repeats gives insight into the mechanism of target recognition. The repeats form an elongated superhilix. The concave surface of the superhelix is lined by absolutely conserved asparagines, in a manner reminiscent of the peptide-binding site of importin alpha. Based on this structural similarity, it is proposed that OGT uses an analogous molecular mechanism to recognize its targets.<ref>PMID:15361863</ref> </StructureSection> | ||
| + | == OGT Mediated Disease == | ||
| + | Faulty regulation of O-GlcNAc modifications has been suggested to be involved in neurodegenerative diseases, diabetes mellitus and cancer. Biochemical details of these processes are still unclear.<ref>PMID:16051707</ref> Proteins modified by O-GlcNAc have been directly shown to have a role in the pathology of human diseases. For instance, the Ser/The kinase AKT,PI<sub>(3)</sub>K,insulin receptor substrate 1, glycogen synthase and endothelial nitric oxide synthase,all of which are enzymes that have a crucial role in insulin signalling,are reversibly modified by OGT. A recent study showed that recruitment of OGT to the plasma membrane specifically prevents the phosphorylation of AKT and possibly other proteins, thereby terminating insulin signalling.<ref>PMID:18288188</ref> This adds evidence to the view that increasing the level of O-GlcNAc modifications correlates with the development of insulin resistance, which is a characteristic of type II diabetes.<ref>PMID:16317114</ref><ref>PMID:16781888</ref> Some indications suggest that O-GlcNAc modifications have a role in Alzheimer disease. Higher levels of O-GlcNAc can be detected in the brain tissue, and several proteins involved in neuronal signaling are modified with O-GlcNAc. Among them are the β-amyloid precursor protein, clathrin-assembly proteins and neurofilaments. In the brains of patients with Alzheimer disease, hyperphosphorylated Tau protein was modified by O-GlcNAc to a lesser extent than in healthy individuals.<ref>PMID:17940659</ref> Studies have shown that some oncogenes and tumour suppressors are targets of O-glycosylation, including the SV40 T antigen and c-MYC.<ref>PMID:14533811</ref> Tumour cells have an altered glucose metabolism that is expected to produce changes in O-GlcNAc levels and to affect different signaling pathways. | ||
==References== | ==References== | ||
<references /> | <references /> | ||
Current revision
| This Sandbox is Reserved from September 14, 2021, through May 31, 2022, for use in the class Introduction to Biochemistry taught by User:John Means at the University of Rio Grande, Rio Grande, OH, USA. This reservation includes 5 reserved sandboxes (Sandbox Reserved 1590 through Sandbox Reserved 1594). |
To get started:
More help: Help:Editing. For an example of a student Proteopedia page, please see Photosystem II, Tetanospasmin, or Guanine riboswitch. |
|
Contents |
O-GlcNAc transferase
O-linked β-N-acetylglucosamine transferase (O-GlcNAc transferase) is an essential mammalian enzyme that acts as a nutrient sensor, coupling metabolic status to the regulation of a wide variety of cellular signaling pathways.[1] OGT catalyses the transfer of N-acetylglucosamine from UDP-N-acetylglucosamine (UDP-GlcNAc) to serines and threonines of cytoplasmic, nuclear and mitochondrial proteins, including numerous transcription factors, tumour suppressors, kinases, phospahateses and histone-modifying proteins.[2] Two crystal structures of human OGT are reported here as a with UDP and a with UDP and a peptide substrate.
O-GlcNAc transferase Function
The major mechanism for nutrient sensing in eukaryotes involves OGT. OGT senses cellular glucose levels via UDP-GlcNAc concentration, and responds by O-GlcNAcylating a broad range of nuclear anf cytoplasmic proteins.[3] Insulin-like signaling pathways and transcriptional activators that regulate glucose levels by controlling gluconeogenisis include proteins that are O-GlcNAcylated by OGT.[4] Numerous O-GlcNAcylation sites are also phosphorylation sites. OGT is suggested to play a major role in modulating cellular kinase signaling cascades.[5] Widespread transcriptional regulations also involve OGT.[6]
OGT Modifications
O-GlcNAc modification has been described for a large and still increasing number of proteins, many of which are key modulators of cellular signalling. O-GlcNAc modifications are catalysed by a OGT, and are removed by the antagonistic enzyme β-N-acetylglucosaminidase (O-GlcNAcase). The general scheme of O-linked N-acetylglucosamine modification suggests that N-acetylglucosamine is added to serine/threonine (Ser/Thr) residues of target proteins by the enzyme OGT, using UDP-GlcNAc as substrate. The N-acetylglucosamine group is removed by the antagonistic activity of O-GlcNAcase.[7]
OGT Features of Interest
The OGT protein possess two ligands and uridine-5-diphosphate . OGT is the only known member to glycosylate polypeptides, and it contains a long uncharacterized intervening sequence (~120 amino acids) in the middle of the catalytic region. Studies suggest that OGT contains a phosphatidylinositol (3,4,5)-trisphosphate (PIP3)binding domain. The most unusual feature of OGT is the intervening domain between the catalytic lobes, which is only found in metazoans. This polypeptide adopts a topologically novel fold with a seven-stranded core stabilized by flanking alpha helices. There are two long for which electron density is missing.[8]
OGT Structure
OGT is comprised of two distinct regions: a multidomain catalytic region, which has no available structure and an N-terminal region consisting of a series of tetratricopeptide repeat(TPR) units.[9] The N terminus of OGT is unusual, consisting of 2.5-13.5 tetratricopeptide repeats (TPRs) depending on alternative splicing.[10] The N-terminal domain of tetratricopeptide (TPR) mediates the recognition of a broad range of target proteins. Components of the nuclear pore complex are major OGT targets, as OGT depletion by RNA interference (RNAi) results in the loss of GlcNAc modification at the nuclear envelope.
N Terminus
| |||||||||||
OGT Mediated Disease
Faulty regulation of O-GlcNAc modifications has been suggested to be involved in neurodegenerative diseases, diabetes mellitus and cancer. Biochemical details of these processes are still unclear.[12] Proteins modified by O-GlcNAc have been directly shown to have a role in the pathology of human diseases. For instance, the Ser/The kinase AKT,PI(3)K,insulin receptor substrate 1, glycogen synthase and endothelial nitric oxide synthase,all of which are enzymes that have a crucial role in insulin signalling,are reversibly modified by OGT. A recent study showed that recruitment of OGT to the plasma membrane specifically prevents the phosphorylation of AKT and possibly other proteins, thereby terminating insulin signalling.[13] This adds evidence to the view that increasing the level of O-GlcNAc modifications correlates with the development of insulin resistance, which is a characteristic of type II diabetes.[14][15] Some indications suggest that O-GlcNAc modifications have a role in Alzheimer disease. Higher levels of O-GlcNAc can be detected in the brain tissue, and several proteins involved in neuronal signaling are modified with O-GlcNAc. Among them are the β-amyloid precursor protein, clathrin-assembly proteins and neurofilaments. In the brains of patients with Alzheimer disease, hyperphosphorylated Tau protein was modified by O-GlcNAc to a lesser extent than in healthy individuals.[16] Studies have shown that some oncogenes and tumour suppressors are targets of O-glycosylation, including the SV40 T antigen and c-MYC.[17] Tumour cells have an altered glucose metabolism that is expected to produce changes in O-GlcNAc levels and to affect different signaling pathways.
References
- ↑ Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature.2007;446:1017-22.[1]
- ↑ Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011 Jan 27;469(7331):564-7. Epub 2011 Jan 16. PMID:21240259 doi:10.1038/nature09638
- ↑ Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007 Apr 26;446(7139):1017-22. PMID:17460662 doi:10.1038/nature05815
- ↑ Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008 Feb 21;451(7181):964-9. PMID:18288188 doi:10.1038/nature06668
- ↑ Cavill I. Reducing blood transfusion. Focus should be on improving patients' ability to make own blood. BMJ. 2002 Sep 21;325(7365):655. PMID:12269319
- ↑ Gambetta MC, Oktaba K, Muller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009 Jul 3;325(5936):93-6. Epub 2009 May 28. PMID:19478141 doi:10.1126/science.1169727
- ↑ Alexander G, Danilo G. The O-linked N-acetylglucosamine modification in cellular signalling and the immune system. EMBO reports. 2008 June;9:748-753[2]
- ↑ Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008 Feb 21;451(7181):964-9. PMID:18288188 doi:10.1038/nature06668
- ↑ Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997 Apr 4;272(14):9308-15. PMID:9083067
- ↑ Kreppel L, Hart G. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015-32022
- ↑ Jinek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004 Oct;11(10):1001-7. Epub 2004 Sep 12. PMID:15361863 doi:10.1038/nsmb833
- ↑ Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11266-71. Epub 2005 Jul 28. PMID:16051707 doi:10.1073/pnas.0408771102
- ↑ Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008 Feb 21;451(7181):964-9. PMID:18288188 doi:10.1038/nature06668
- ↑ Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the "O-GlcNAc code". Sci STKE. 2005 Nov 29;2005(312):re13. PMID:16317114 doi:10.1126/stke.3122005re13
- ↑ Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006 May-Jun;1761(5-6):599-617. Epub 2006 May 6. PMID:16781888 doi:10.1016/j.bbalip.2006.04.007
- ↑ Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer's disease. Mol Biosyst. 2007 Nov;3(11):766-72. Epub 2007 Aug 29. PMID:17940659 doi:10.1039/b704905f
- ↑ Chou TY, Hart GW. O-linked N-acetylglucosamine and cancer: messages from the glycosylation of c-Myc. Adv Exp Med Biol. 2001;491:413-8. PMID:14533811