User:Brian Hernandez/DOPA Decarboxylase
From Proteopedia
< User:Brian Hernandez(Difference between revisions)
| (18 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='1js3' size=' | + | <StructureSection load='1js3' size='600' side='right' caption='DDC in complex with carbiDOPA (PDB entry [[1js3]])' scene=''> |
==Introduction== | ==Introduction== | ||
---- | ---- | ||
| - | DOPA decarboxylase (Aromatic L-Amino Acid Decarboxylase, tryptophan decarboxylase, 5-hydroxytryptophan decarboxylase, AAAD, or | + | DOPA decarboxylase (Aromatic L-Amino Acid Decarboxylase, tryptophan decarboxylase, 5-hydroxytryptophan decarboxylase, AAAD, or [[DDC]]) is an essential [http://en.wikipedia.org/wiki/Lyase lyase] enzyme responsible for the conversion (via decarboxylation) of L-3,4-dihydroxyphenylalanine (L-DOPA) and L-5-hydroxytryptophan (5-HTP) to [http://en.wikipedia.org/wiki/Dopamine dopamine] and [http://en.wikipedia.org/wiki/Serotonin serotonin]<ref name=Christenson>PMID: 4536745 </ref>. This 104 kDa protein is a tightly associated α<sub>2</sub>-dimer that belongs to the '''aspartate aminotransferase family''' (fold type 1) of PLP-dependent (vitamin B6-dependent) enzymes, with the catalytically active form of the enzyme existing as the homodimer<ref name="schneider">PMID:10673430 </ref>. DDC can be found in abundance in the nervous system, as well as the kidney. Because of its role in the biosynthesis of dopamine, DDC has been utilized in the treatment of [http://en.wikipedia.org/wiki/Parkinson's_disease Parksinon's disease]- a chronic, progressively neurological disorder, thought to be the result of degeneration of dopamine-producing cells in the '''substantia nigra pars compacta''' (brain structure in the mesencephalon that plays an important role in reward, addiction, and movement) of the brain. |
==Structure== | ==Structure== | ||
---- | ---- | ||
| - | DDC consists of two monomers | + | DDC consists of two monomers, each with three distinct domains: the large domain, the C-terminal small domain, and the N-terminal domain <ref name=Burkhard>PMID: 11685243 </ref>. The <scene name='DOPA_decarboxylase/Large_domain/1'>large domain</scene> consists of the cofactor (PLP- pyridoxal phosphate) binding site and is comprised of a central, seven-stranded mixed β-sheet surrounded by eight α-helices in a typical α/β fold. The small <scene name='DOPA_decarboxylase/Small_domain/1'>C-terminal domain</scene> consists of a four-stranded antiparallel β-sheet with three helices packed against the face opposite the large domain. The <scene name='Sandbox/N-terminal_domain/2'>N-terminal domain</scene> (characteristic of all α-family enzymes) is composed of two parallel helices linked by an extended strand. This structure flaps over the top of the second subunit and vice versa, with the first helix of one subunit aligning parallel to the equivalent helix of the other subunit, thereby forming the '''extended dimer interface'''. Although it is unlikely that this domain represents an autonomous folding unit, it is most likely stable only in the context of the dimer by extending the interface between the two monomers. |
==Function== | ==Function== | ||
==='''The Active Site'''=== | ==='''The Active Site'''=== | ||
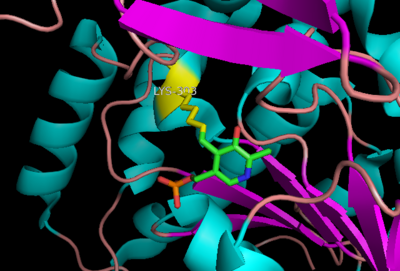
| - | DDC's active site is located in a cleft between the two monomer subunits, but is composed mainly of residues from one monomer.The active site is composed of several key residues, including Lys-303, Asp-271, His-192, Thr-82, Ile-101, and Phe-103. In the ligand free form, PLP binds to Lys 303 via a Schiff base linkage. A salt bridge forms between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP yielding a strong electron sink capable of stabilizing the carbanionic intermediates. The only two active site residues from the adjacent monomer, Ile-101 and Phe-103, are part of the substrate binding pocket. | + | DDC's active site is located in a <scene name='DOPA_decarboxylase/Dimer_interface/2'>cleft</scene> between the two monomer subunits, but is composed mainly of residues from one monomer.The <scene name='DOPA_decarboxylase/Active_site/1'>active site</scene> is composed of several key residues, including Lys-303, Asp-271, His-192, Thr-82, Ile-101, and Phe-103. In the ligand free form, PLP binds to Lys 303 via a '''Schiff base linkage'''. A [http://en.wikipedia.org/wiki/Salt_bridge_(protein) salt bridge] forms between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP yielding a strong '''electron sink''' capable of stabilizing the carbanionic intermediates. The only two active site residues from the adjacent monomer, Ile-101 and Phe-103, are part of the substrate binding pocket that stabilizes the substrate via <scene name='DOPA_decarboxylase/Vanderwaals/1'>van der waals forces</scene>. |
| + | [[image:plp bound.png|thumb|center|400px|'''Schiff base linkage of PLP to Lys303 in the active site''']] | ||
==='''Flexible Loop'''=== | ==='''Flexible Loop'''=== | ||
| - | Residues 328-338 are a stretch of 11 amino acids that comprise a mobile loop (located near the dimer interface), thought to be significant in the catalytic mechanism. During catalysis, the loop is expected to adhere to a less solvent- and protease-exposed conformation, whereby it loses flexibility and extends toward the active site. Tyr-332 and Lys-334 are highly conserved residues indicative of the flexible loop's possible role in catalysis. The conformational change that occurs is thought to be a result of loop residues directly interacting with the inhibitor. Finally, with such a conformational change, the Tyr-332 residue could then be located closer to the substrate to possibly act as a proton donor for the quinonoid Cα. | + | Residues 328-338 are a stretch of 11 amino acids that comprise a mobile loop (located near the dimer interface), thought to be significant in the catalytic mechanism<ref name=Ishii>PMID: 8889823 </ref>. During catalysis, the loop is expected to adhere to a less solvent- and protease-exposed conformation, whereby it loses flexibility and extends toward the active site. Tyr-332 and Lys-334 are highly conserved residues indicative of the flexible loop's possible role in catalysis. The conformational change that occurs is thought to be a result of loop residues directly interacting with the inhibitor. Finally, with such a conformational change, the Tyr-332 residue could then be located closer to the substrate to possibly act as a proton donor for the quinonoid Cα. |
| - | + | ||
| - | + | ||
==DDC and Parkinson's Disease== | ==DDC and Parkinson's Disease== | ||
==='''Treatment'''=== | ==='''Treatment'''=== | ||
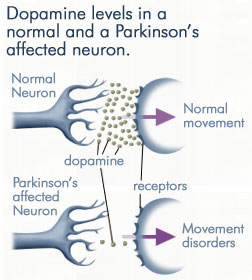
| - | Parkinson's disease | + | Parkinson's disease can be characterized by [http://en.wikipedia.org/wiki/Tremor tremor], [http://en.wikipedia.org/wiki/Bradykinesia#Bradykinesia bradykinesia], rigidity, and postural instability. [[Image:dopamine levels.jpeg|thmb|right|300px|'''Dopamine Levels''']]With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier<ref name=Burkhard>PMID: 11685243 </ref>. However, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly converted to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and reducing the side effects of dopamine-rich blood. |
==='''Inhibitor Binding'''=== | ==='''Inhibitor Binding'''=== | ||
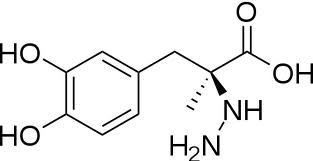
| - | The inhibitor '' | + | The inhibitor <scene name='DOPA_decarboxylase/Carbidopa/1'>carbiDOPA</scene> binds to the enzyme by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety <ref name=Burkhard>PMID: 11685243 </ref>. |
| - | [[Image:carbidopa.jpeg|thumb|right|400px|'''carbiDOPA''']] | + | [[Image:carbidopa.jpeg|thumb|right|400px|'''carbiDOPA''']] The catechol ring buries itself deep within the active site cleft, with the 4' hydroxyl of the catechol ring hydrogen bonded to the hydroxyl group of Thr 82. Additionally, the highly conserved His 192 residue forms a hydrogen bond to the carboxylate group of carbiDOPA (with the carboxylate group of L-DOPA being even closer in binding range). Thus, His 192 is most likely a key residue in the enzyme's catalytic activity because a H192A mutation can cause complete loss of activity<ref name=Burkhard>PMID: 11685243 </ref>. |
| + | </StructureSection> | ||
| + | |||
| + | ==3D structures of DOPA decarboxylase== | ||
| + | |||
| + | {{STRUCTURE_1js3| PDB=1js3 | SIZE=250| SCENE= }} | ||
| + | |||
| + | [[3k40]] – DDC – ''Drosophila melanogaster''<br /> | ||
| + | [[1js3]] – pDDC + inhibitor – pig<br /> | ||
| + | [[1js6]] - pDDC<br /> | ||
| + | [[3rbf]], [[3rbl]] – hDDC – human<br /> | ||
| + | [[3rch]] – hDDC + vitamin B6 phosphate + pyridoxal phosphate | ||
| + | |||
| + | ==References== | ||
| + | |||
| + | {{Reflist}} | ||
Current revision
| |||||||||||
3D structures of DOPA decarboxylase
| |||||||||
| 1js3, resolution 2.25Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| Activity: | Aromatic-L-amino-acid decarboxylase, with EC number 4.1.1.28 | ||||||||
| Related: | 1js6 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
3k40 – DDC – Drosophila melanogaster
1js3 – pDDC + inhibitor – pig
1js6 - pDDC
3rbf, 3rbl – hDDC – human
3rch – hDDC + vitamin B6 phosphate + pyridoxal phosphate
References
- ↑ Christenson JG, Dairman W, Udenfriend S. On the identity of DOPA decarboxylase and 5-hydroxytryptophan decarboxylase (immunological titration-aromatic L-amino acid decarboxylase-serotonin-dopamine-norepinephrine). Proc Natl Acad Sci U S A. 1972 Feb;69(2):343-7. PMID:4536745
- ↑ Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000 Jan 15;8(1):R1-6. PMID:10673430
- ↑ 3.0 3.1 3.2 3.3 Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243 doi:http://dx.doi.org/10.1038/nsb1101-963
- ↑ Ishii S, Mizuguchi H, Nishino J, Hayashi H, Kagamiyama H. Functionally important residues of aromatic L-amino acid decarboxylase probed by sequence alignment and site-directed mutagenesis. J Biochem. 1996 Aug;120(2):369-76. PMID:8889823