We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
ExbD
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='2pfu' size='350' side='right' scene='' caption=''> | ||
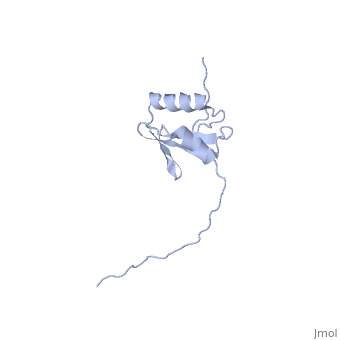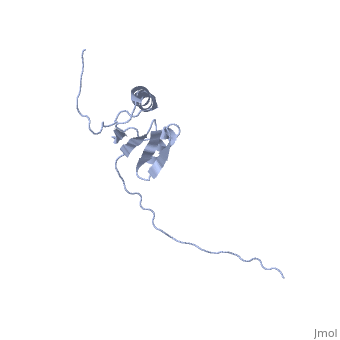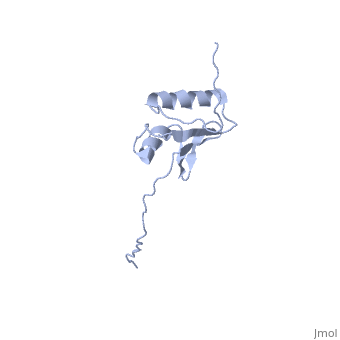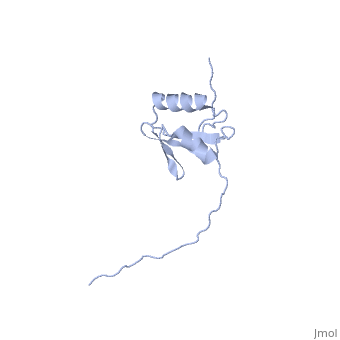
[[Image:ExbD.jpg|300px|left|thumb| The Structure of ExbD<ref name='Kampfenkel'>PMID: 1644779</ref>]] | [[Image:ExbD.jpg|300px|left|thumb| The Structure of ExbD<ref name='Kampfenkel'>PMID: 1644779</ref>]] | ||
==Structure== | ==Structure== | ||
| - | + | ||
'''ExbD''' has a single transmembrane domain, with residues 1 to 22 on the cytoplasmic side and 44 to 141 in the periplasm (''see'' 3D structure 2PFU). Residues 23 to 43 are within the cytoplasmic membrane and it is in this region, from residues 18 to 43, that the only hydrophobic residues in ExbD can be found<ref name='Kampfenkel'>PMID: 1644779</ref>. | '''ExbD''' has a single transmembrane domain, with residues 1 to 22 on the cytoplasmic side and 44 to 141 in the periplasm (''see'' 3D structure 2PFU). Residues 23 to 43 are within the cytoplasmic membrane and it is in this region, from residues 18 to 43, that the only hydrophobic residues in ExbD can be found<ref name='Kampfenkel'>PMID: 1644779</ref>. | ||
| Line 12: | Line 13: | ||
The activity of ExbD can be affected with mutations of the single charged amino acid (here D25N) which lies close to the transmembrane region. This can also be said of the other transmembrane proteins ExbB, TolQ and TolR. | The activity of ExbD can be affected with mutations of the single charged amino acid (here D25N) which lies close to the transmembrane region. This can also be said of the other transmembrane proteins ExbB, TolQ and TolR. | ||
| + | </StructureSection> | ||
| + | ==3D structures of ExbD== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | + | [[2pfu]] – EcExbD periplasmic domain – ''Escherichia coli'' – NMR<br /> | |
| - | + | [[5sv1]] – EcExbD residues 1-49 + EcExbB<br /> | |
| - | [[2pfu]] – | + | [[6tyi]] – EcExbD + ExbB – Cryo EM<br /> |
| - | + | [[8pek]] – SmExbD periplasmic domain 43-140 - ''Serratia marsescens'' - NMR<br /> | |
| + | [[8p9r]] – SmExbD periplasmic domain + TonB<br /> | ||
| + | [[8vgc]], [[8vgd]] – SmExbD periplasmic domain + D-box peptide<br /> | ||
| + | [[7ajq]] – SmExbD + ExbB - Cryo EM<br /> | ||
== References== | == References== | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of ExbD
Updated on 03-June-2025
2pfu – EcExbD periplasmic domain – Escherichia coli – NMR
5sv1 – EcExbD residues 1-49 + EcExbB
6tyi – EcExbD + ExbB – Cryo EM
8pek – SmExbD periplasmic domain 43-140 - Serratia marsescens - NMR
8p9r – SmExbD periplasmic domain + TonB
8vgc, 8vgd – SmExbD periplasmic domain + D-box peptide
7ajq – SmExbD + ExbB - Cryo EM
References
- ↑ 1.0 1.1 1.2 Kampfenkel K, Braun V. Membrane topology of the Escherichia coli ExbD protein. J Bacteriol. 1992 Aug;174(16):5485-7. PMID:1644779
- ↑ Held KG, Postle K. ExbB and ExbD do not function independently in TonB-dependent energy transduction. J Bacteriol. 2002 Sep;184(18):5170-3. PMID:12193634
- ↑ Braun V, Herrmann C. Point mutations in transmembrane helices 2 and 3 of ExbB and TolQ affect their activities in Escherichia coli K-12. J Bacteriol. 2004 Jul;186(13):4402-6. PMID:15205446 doi:10.1128/JB.186.13.4402-4406.2004