We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Human lactoferrin
From Proteopedia
(Difference between revisions)
(Undo revision 3437870 by Karsten Theis (Talk)) |
|||
| (12 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1dsn' size='450' side='right' scene='' caption='Human lactoferrin complex with Fe and carbonate, [[1dsn]]'> | |
| - | {{STRUCTURE_1dsn|PDB=1dsn|SCENE=}} | ||
=Amino-Terminal Half-Molecule of Human Lactoferrin= | =Amino-Terminal Half-Molecule of Human Lactoferrin= | ||
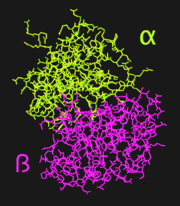
Human lactoferrin, LF, is a protein in the transferrin family. As such, it has the ability to tightly bind iron in conjunction with a large-scale conformational change associated with iron binding and release.<ref name="faber">PMID:8594202</ref> These properties give lactoferrin the ability to regulate iron, and possibly other metal, ion levels in the fluids and secretions, such as milk, of animals.<ref name="faber" /> Lactoferrin is folded into two lobes: the N-terminal half, LF<sub>N</sub> ([[1dsn]]), and the C-terminal half, LF<sub>C</sub>. The two LF lobes have 37% homology and very similar tertiary structures; it has been suggested that the two lobes are the product of gene duplication.<ref name="farnaud" /> Each lobe of LF<sub>N</sub> is further subdivided into two similarly sized α and β domains (Figure 1); the <scene name='Sandbox_Reserved_302/Ligand_site/1'>iron binding site</scene> is situated in a deep cleft between the two domains.<ref name="faber" /> | Human lactoferrin, LF, is a protein in the transferrin family. As such, it has the ability to tightly bind iron in conjunction with a large-scale conformational change associated with iron binding and release.<ref name="faber">PMID:8594202</ref> These properties give lactoferrin the ability to regulate iron, and possibly other metal, ion levels in the fluids and secretions, such as milk, of animals.<ref name="faber" /> Lactoferrin is folded into two lobes: the N-terminal half, LF<sub>N</sub> ([[1dsn]]), and the C-terminal half, LF<sub>C</sub>. The two LF lobes have 37% homology and very similar tertiary structures; it has been suggested that the two lobes are the product of gene duplication.<ref name="farnaud" /> Each lobe of LF<sub>N</sub> is further subdivided into two similarly sized α and β domains (Figure 1); the <scene name='Sandbox_Reserved_302/Ligand_site/1'>iron binding site</scene> is situated in a deep cleft between the two domains.<ref name="faber" /> | ||
| Line 9: | Line 8: | ||
=Structure= | =Structure= | ||
[[Image:1DSN_Domains.png|left|thumb|'''Figure 1.''' Cartoon illustrating the alpha (green) and beta (magenta) domains of LF<sub>N</sub>]] | [[Image:1DSN_Domains.png|left|thumb|'''Figure 1.''' Cartoon illustrating the alpha (green) and beta (magenta) domains of LF<sub>N</sub>]] | ||
| + | {{Clear}} | ||
The amino-terminal half-molecule of human lactoferrin (LF<sub>N</sub>) is comprised of a single 333 amino acid chain divided into two similarly-sized α and β domains. The iron binding site is located within a deep cleft between the lobes, where iron is bound by <scene name='Sandbox_Reserved_302/Helix_3_and_5/2'>Helices 3 and 5</scene> of the α and β domains, respectively. Iron, which is bound to a carboxylate ion, is bound by Asp60, Ala123, and Gly124. Although unwound in LF<sub>N</sub>, <scene name='Sandbox_Reserved_302/Pigtail/1'>residues 313 to 333</scene> form a helix when joined to LF<sub>C</sub>, forming the full LF protein.<ref name="faber" /> | The amino-terminal half-molecule of human lactoferrin (LF<sub>N</sub>) is comprised of a single 333 amino acid chain divided into two similarly-sized α and β domains. The iron binding site is located within a deep cleft between the lobes, where iron is bound by <scene name='Sandbox_Reserved_302/Helix_3_and_5/2'>Helices 3 and 5</scene> of the α and β domains, respectively. Iron, which is bound to a carboxylate ion, is bound by Asp60, Ala123, and Gly124. Although unwound in LF<sub>N</sub>, <scene name='Sandbox_Reserved_302/Pigtail/1'>residues 313 to 333</scene> form a helix when joined to LF<sub>C</sub>, forming the full LF protein.<ref name="faber" /> | ||
The structure of LF<sub>N</sub> undergoes a dramatic conformational change upon iron binding. Upon iron binding, the two domains of LF<sub>N</sub> undergo a rigid 54.1º rotation about a <scene name='Sandbox_Reserved_302/Hinge/1'>screw axis</scene> that passes through Thr90 and Pro251.<ref name="gerstein">PMID:8230220</ref> | The structure of LF<sub>N</sub> undergoes a dramatic conformational change upon iron binding. Upon iron binding, the two domains of LF<sub>N</sub> undergo a rigid 54.1º rotation about a <scene name='Sandbox_Reserved_302/Hinge/1'>screw axis</scene> that passes through Thr90 and Pro251.<ref name="gerstein">PMID:8230220</ref> | ||
| + | |||
| + | To visualize the <jmol> | ||
| + | <jmolLink> | ||
| + | <script>load files "=1CB6" "=1LCF";model 0;cartoon only;domain2 = {(92-249 or 1092-1249)};select domain2 and _C;color green; | ||
| + | structures = [{1.1}, {2.1}]; | ||
| + | domains = [[{not domain2},{not domain2}, {alpha and not domain2 and not altloc="B"}], [{domain2}, {(91,1091,250,1250) and *.CA}, {alpha and domain2 and not altloc="B"}],];moveto 1.0 { 880 471 63 56.8} 132.25 0.0 0.0 {2.646000000000001 15.972 -7.2715} 63.954979842981245 {0 0 0} 0 0 0 3.0 0.0 0.0; | ||
| + | script "https://proteopedia.org/wiki/images/a/a2/Storymorph.spt";superimpose(structures,domains,1);model 1;delay 0.5;model 2;delay 0.5;model 1; | ||
| + | </script> | ||
| + | <text>domain motion</text> | ||
| + | </jmolLink> | ||
| + | </jmol>, we first load the two structures and superimpose them. You can choose a viewing orientation before pressing the morph button, which will visualize the conformational change from structure 1CB6 to 1LCF and back again<ref>The [[Jmol/Storymorph|Storymorph Jmol scripts]] creates the interpolated coordinates of the morph on the fly.</ref> | ||
| + | . | ||
| + | |||
| + | <jmol> | ||
| + | <jmolButton> | ||
| + | <script>morph_palindrome = 1; | ||
| + | morph(15,structures,domains)</script> | ||
| + | <text>Morph</text> | ||
| + | </jmolButton> | ||
| + | </jmol> <jmol> | ||
| + | <jmolButton> | ||
| + | <script>select protein;spacefill only;</script> | ||
| + | <text>spacefill</text> | ||
| + | </jmolButton> | ||
| + | </jmol> | ||
| + | |||
| + | |||
=Function & Application= | =Function & Application= | ||
| Line 24: | Line 51: | ||
[[Lactoferrin]] | [[Lactoferrin]] | ||
| - | + | </StructureSection> | |
=References= | =References= | ||
<references /> | <references /> | ||
| - | Page originally authored by Christian Axen | + | Page originally authored by [http://proteopedia.org/wiki/index.php/User:Christian_Axen Christian Axen] |
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Faber HR, Bland T, Day CL, Norris GE, Tweedie JW, Baker EN. Altered domain closure and iron binding in transferrins: the crystal structure of the Asp60Ser mutant of the amino-terminal half-molecule of human lactoferrin. J Mol Biol. 1996 Feb 23;256(2):352-63. PMID:8594202
- ↑ 2.0 2.1 2.2 Farnaud S, Evans RW. Lactoferrin--a multifunctional protein with antimicrobial properties. Mol Immunol. 2003 Nov;40(7):395-405. PMID:14568385
- ↑ 3.0 3.1 3.2 Sanchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. 1992 May;67(5):657-61. PMID:1599309
- ↑ Gerstein M, Anderson BF, Norris GE, Baker EN, Lesk AM, Chothia C. Domain closure in lactoferrin. Two hinges produce a see-saw motion between alternative close-packed interfaces. J Mol Biol. 1993 Nov 20;234(2):357-72. PMID:8230220 doi:http://dx.doi.org/10.1006/jmbi.1993.1592
- ↑ The Storymorph Jmol scripts creates the interpolated coordinates of the morph on the fly.
- ↑ 6.0 6.1 van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK. Antiviral activities of lactoferrin. Antiviral Res. 2001 Dec;52(3):225-39. PMID:11675140
Page originally authored by Christian Axen
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Alexander Berchansky, Michal Harel, Andrea Gorrell