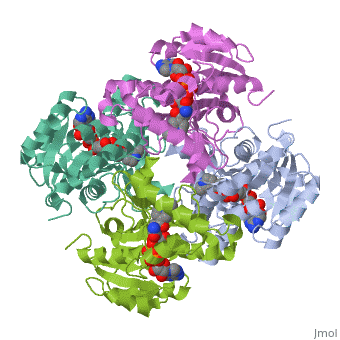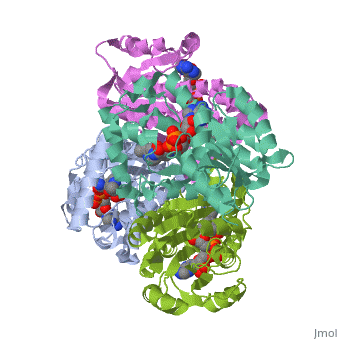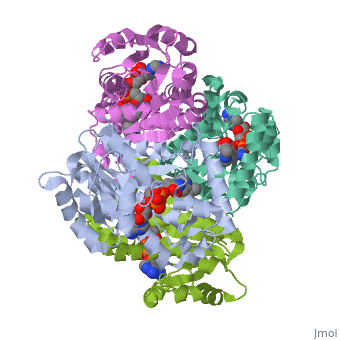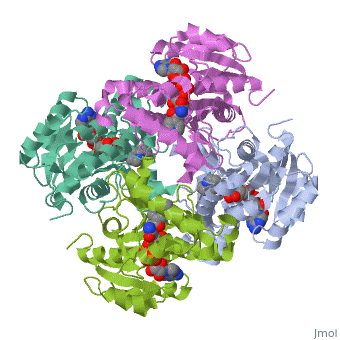
We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
InhA
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='2h9i' size='350' side='right' scene='' caption='Enoyl-[acyl-carrier-protein] reductase complex with ETH-NAD (PDB code [[2h9i]])'> | ||
='''InhA'''= | ='''InhA'''= | ||
| - | |||
by Kelly Hrywkiw | by Kelly Hrywkiw | ||
| - | {{STRUCTURE_2h9i | PDB=2h9i | SCENE= }} | ||
__TOC__ | __TOC__ | ||
| - | |||
| - | |||
| - | |||
=Introduction= | =Introduction= | ||
| - | The enzyme InhA is coded from the INHA gene that is similar in sequence to the ''[http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]''gene which plays a role in [http://en.wikipedia.org/wiki/Fatty_acid_synthesis fatty acid synthesis], and is part of a short chain dehydrogenase/reductase family<ref name ="making drugs for inhA">Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. Inha is an [http://en.wikipedia.org/wiki/NADH NADH] dependent trans enoyl-acyl ACP carrier protein that is part of the fatty acid biosynthesis system: fatty acid synthase two (FASII), and plays a role in the synthesis of [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic Acid]<ref name ="mech of thioamide drug action">PMID:17227913</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. Mycolic acids are long chain fatty acids (C54 to C63) that are essential in cell wall formation of the human pathogen ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]''as well as other mycobateria such as ''[http://en.wikipedia.org/wiki/Mycobacterium_leprae Mycobacterium leprae]'', and are associated with virulence<ref name ="TB">PMID2568869:</ref>. InhA has been proposed as the target of the [http://en.wikipedia.org/wiki/Thioamidedrugs thioamide] drugs, ethionamide (ETH) and protionamide (PTH), which have been used in treatment of mycobacterial infections <ref name ="phosphorylation of inhA">PMID:21143326</ref>. However stains of ''M. tuberculosis'' that are resistant to thioamide drugs have been increaseing worldwide, and therefor research into the exact mechanisms of these drugs is of importance. | + | The enzyme''' InhA''' is coded from the INHA gene that is similar in sequence to the ''[http://en.wikipedia.org/wiki/Salmonella_typhimurium Salmonella typhimurium]'' gene which plays a role in [http://en.wikipedia.org/wiki/Fatty_acid_synthesis fatty acid synthesis], and is part of a short chain dehydrogenase/reductase family<ref name ="making drugs for inhA">Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. Inha is an [http://en.wikipedia.org/wiki/NADH NADH] dependent trans enoyl-acyl ACP carrier protein that is part of the fatty acid biosynthesis system: fatty acid synthase two (FASII), and plays a role in the synthesis of [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic Acid]<ref name ="mech of thioamide drug action">PMID:17227913</ref><ref name ="phosphorylation of inhA">PMID:21143326</ref>. Mycolic acids are long chain fatty acids (C54 to C63) that are essential in cell wall formation of the human pathogen ''[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis]''as well as other mycobateria such as ''[http://en.wikipedia.org/wiki/Mycobacterium_leprae Mycobacterium leprae]'', and are associated with virulence<ref name ="TB">PMID2568869:</ref>. InhA has been proposed as the target of the [http://en.wikipedia.org/wiki/Thioamidedrugs thioamide] drugs, ethionamide (ETH) and protionamide (PTH), which have been used in treatment of mycobacterial infections <ref name ="phosphorylation of inhA">PMID:21143326</ref>. However stains of ''M. tuberculosis'' that are resistant to thioamide drugs have been increaseing worldwide, and therefor research into the exact mechanisms of these drugs is of importance. |
=Structure of InhA= | =Structure of InhA= | ||
| - | [[Image:Stero veiw.png|thumb| | + | <scene name='Sandbox_Reserved_321/Structural_progresion/1'>Momomeric subunit of InhA with bound EAD</scene> |
| + | [[Image:Stero veiw.png|thumb|left|upright=2.5|alt=Secondary Structure Succession of InhA. Secondary structure residues are ordered from blue to red.|Fig.1: Stero view of the homotetramer structure of InhA with secondary structure succession outlined]] | ||
| - | + | The InhA enzyme of ''M. tuberculosis'' is a homotetramer (Fig. 1) composed of a repeating subunit of a single domain with a [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann Fold] in the core that provides a NADH binding site<ref name ="crystallographic studies"/>. The single domain can be broken down into two substructures that are connected by short peptide loop<ref name ="making drugs for inhA"/><ref name ="crystallographic studies">PMID:17588773</ref>. The overall structure exhibits α/β folding with a series of [http://en.wikipedia.org/wiki/Alpha_helix α helices] flanking a central [http://en.wikipedia.org/wiki/Beta_sheet β sheet] of multiple parallel β strands<ref name ="crystallographic studies"/>. | |
| - | + | ||
| - | The InhA enzyme | + | |
==Substructure 1 of InhA== | ==Substructure 1 of InhA== | ||
| Line 36: | Line 31: | ||
=InhA's Function in the Mycolic Acid Pathway= | =InhA's Function in the Mycolic Acid Pathway= | ||
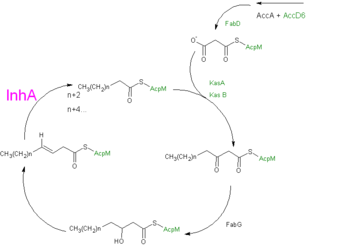
| - | [[Image:Pathway2.png|thumb| | + | [[Image:Pathway2.png|thumb|left|upright=2|alt=Proposed mechanism.|Fig 2: Formulated mechanism of Mycolic acid synthesis as proposed by Wilson et al.<ref name ="Drug Induced Alterations">PMID:10536008</ref>.]] |
| - | + | {{Clear}} | |
InhA plays a key role in the synthesis of fatty acids, particularly in ''M. tuberculosis'' which, has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together function in the synthesis of mycolic acids<ref name ="Function of M Tb">PMID:18552191</ref>. FASI synthesizes C16-18 and C24-26 fatty acids. The fatty acids from FASI are then sent to FASII which promotes chain extension, forming long-chain meromycolic acids that are 56-64 carbons in length<ref name ="Fatty Acid Synthesis">PMID:18804030</ref>. The final step in FASII is completed by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation(Fig. 2)<ref name ="Function of M Tb"/><ref name ="Roles of T158">PMID:10521269</ref>. | InhA plays a key role in the synthesis of fatty acids, particularly in ''M. tuberculosis'' which, has type one fatty acid synthesis (FASI) and type two fatty acid synthesis (FASII) which together function in the synthesis of mycolic acids<ref name ="Function of M Tb">PMID:18552191</ref>. FASI synthesizes C16-18 and C24-26 fatty acids. The fatty acids from FASI are then sent to FASII which promotes chain extension, forming long-chain meromycolic acids that are 56-64 carbons in length<ref name ="Fatty Acid Synthesis">PMID:18804030</ref>. The final step in FASII is completed by InhA which reduces 2-trans-enoyl-ACP's with chain lengths over twelve carbons in a NADP dependent manner where the hydride transfer precedes protonation(Fig. 2)<ref name ="Function of M Tb"/><ref name ="Roles of T158">PMID:10521269</ref>. | ||
| Line 44: | Line 39: | ||
=InhA and Thioamide Drugs= | =InhA and Thioamide Drugs= | ||
| - | + | [[Image:ETH, EAD, PTH, P1H structures.png|thumb|left|upright=1.5|alt=ETH, EAD, PTH, and P1H.|Fig 3: Structures of ETH, EAD, PTH, and P1H]] | |
| - | + | ||
| - | [[Image:ETH, EAD, PTH, P1H structures.png|thumb| | + | |
The primary target of the thioamide drugs PTH and ETH have been shown to be InhA <scene name='Sandbox_Reserved_321/Structural_progresion/1'>(go to original scene)</scene> in both genetic and molecular experiments<ref name ="mech of thioamide drug action"/>. Both PTH and ETH require activation by various cellular componets to form the NAD adduct that acts to inhibit InhA, and therefore connot be studied in [http://en.wikipedia.org/wiki/In_vitro in vitro]<ref name ="mech of thioamide drug action"/>. The exacct mechanism of their activation is still under speculation, however a flavin monooxygenase (EthA) has been shown to participate in ETH and PTH activation<ref name ="mech of thioamide drug action"/>. In fact, strains of ''M. tuberculosis'' that have mutations in the gene which express EthA exhibit resistance to thioamide drugs<ref name ="mech of thioamide drug action"/>. Currently studies are being carried out to determine other methods of treatment for mycobaterial infections that dont require activation by cellular constituents, due to the incerease of drug resistant cases world wide. | The primary target of the thioamide drugs PTH and ETH have been shown to be InhA <scene name='Sandbox_Reserved_321/Structural_progresion/1'>(go to original scene)</scene> in both genetic and molecular experiments<ref name ="mech of thioamide drug action"/>. Both PTH and ETH require activation by various cellular componets to form the NAD adduct that acts to inhibit InhA, and therefore connot be studied in [http://en.wikipedia.org/wiki/In_vitro in vitro]<ref name ="mech of thioamide drug action"/>. The exacct mechanism of their activation is still under speculation, however a flavin monooxygenase (EthA) has been shown to participate in ETH and PTH activation<ref name ="mech of thioamide drug action"/>. In fact, strains of ''M. tuberculosis'' that have mutations in the gene which express EthA exhibit resistance to thioamide drugs<ref name ="mech of thioamide drug action"/>. Currently studies are being carried out to determine other methods of treatment for mycobaterial infections that dont require activation by cellular constituents, due to the incerease of drug resistant cases world wide. | ||
| Line 81: | Line 74: | ||
InhA can be further classified into the acyl carrier protein family(ACP's). These proteins generally all function in the transport of substrates in a myriad of pathways, such as: the synthesis of polypeptides and fatty acids<ref name ="Acyl Carrier Proteins">PMID:17012233</ref>. | InhA can be further classified into the acyl carrier protein family(ACP's). These proteins generally all function in the transport of substrates in a myriad of pathways, such as: the synthesis of polypeptides and fatty acids<ref name ="Acyl Carrier Proteins">PMID:17012233</ref>. | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
''3D structures of ACP family'' | ''3D structures of ACP family'' | ||
| - | [[Enoyl-Acyl-Protein Reductase | + | [[Enoyl-Acyl-Carrier Protein Reductase]] |
| + | |||
=Additional Resources= | =Additional Resources= | ||
*[http://www.pdb.org/pdb/explore/explore.do?structureId=2H9I Mycobacterium tuberculosis InhA bound with ETH-NAD adduct, in the RCSB Protein Data Bank] | *[http://www.pdb.org/pdb/explore/explore.do?structureId=2H9I Mycobacterium tuberculosis InhA bound with ETH-NAD adduct, in the RCSB Protein Data Bank] | ||
Current revision
| |||||||||||
3D structures of ACP family
Enoyl-Acyl-Carrier Protein Reductase
Additional Resources
- Mycobacterium tuberculosis InhA bound with ETH-NAD adduct, in the RCSB Protein Data Bank
- Mycobacterium tuberculosis InhA bound with PTH-NAD adduct, in the RCSB Protein Data Bank
- Crystal structure of wild-type InhA:NADH complex, in the RCSB Protein Data Bank
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Sacchettini, James (New Rochelle, NY) 1999 INHA crystals and three dimensional structure United States Albert Einstein College of Medicine of Yeshiva University (Bronx, NY) 5882878 http://www.freepatentsonline.com/5882878.html
- ↑ 2.0 2.1 2.2 Molle V, Gulten G, Vilcheze C, Veyron-Churlet R, Zanella-Cleon I, Sacchettini JC, Jacobs WR Jr, Kremer L. Phosphorylation of InhA inhibits mycolic acid biosynthesis and growth of Mycobacterium tuberculosis. Mol Microbiol. 2010 Dec;78(6):1591-605. doi:, 10.1111/j.1365-2958.2010.07446.x. Epub 2010 Nov 9. PMID:21143326 doi:10.1111/j.1365-2958.2010.07446.x
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Wang F, Langley R, Gulten G, Dover LG, Besra GS, Jacobs WR Jr, Sacchettini JC. Mechanism of thioamide drug action against tuberculosis and leprosy. J Exp Med. 2007 Jan 22;204(1):73-8. Epub 2007 Jan 16. PMID:17227913 doi:10.1084/jem.20062100
- ↑ . PMID:216315890657
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Dias MV, Vasconcelos IB, Prado AM, Fadel V, Basso LA, de Azevedo WF Jr, Santos DS. Crystallographic studies on the binding of isonicotinyl-NAD adduct to wild-type and isoniazid resistant 2-trans-enoyl-ACP (CoA) reductase from Mycobacterium tuberculosis. J Struct Biol. 2007 Sep;159(3):369-80. Epub 2007 May 3. PMID:17588773 doi:http://dx.doi.org/10.1016/j.jsb.2007.04.009
- ↑ 6.0 6.1 Rozwarski DA, Vilcheze C, Sugantino M, Bittman R, Sacchettini JC. Crystal structure of the Mycobacterium tuberculosis enoyl-ACP reductase, InhA, in complex with NAD+ and a C16 fatty acyl substrate. J Biol Chem. 1999 May 28;274(22):15582-9. PMID:10336454
- ↑ Wilson M, DeRisi J, Kristensen HH, Imboden P, Rane S, Brown PO, Schoolnik GK. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12833-8. PMID:10536008
- ↑ 8.0 8.1 Gurvitz A, Hiltunen JK, Kastaniotis AJ. Function of heterologous Mycobacterium tuberculosis InhA, a type 2 fatty acid synthase enzyme involved in extending C20 fatty acids to C60-to-C90 mycolic acids, during de novo lipoic acid synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol. 2008 Aug;74(16):5078-85. Epub 2008 Jun 13. PMID:18552191 doi:10.1128/AEM.00655-08
- ↑ 9.0 9.1 Bhatt A, Brown AK, Singh A, Minnikin DE, Besra GS. Loss of a mycobacterial gene encoding a reductase leads to an altered cell wall containing beta-oxo-mycolic acid analogs and accumulation of ketones. Chem Biol. 2008 Sep 22;15(9):930-9. PMID:18804030 doi:10.1016/j.chembiol.2008.07.007
- ↑ 10.0 10.1 10.2 Parikh S, Moynihan DP, Xiao G, Tonge PJ. Roles of tyrosine 158 and lysine 165 in the catalytic mechanism of InhA, the enoyl-ACP reductase from Mycobacterium tuberculosis. Biochemistry. 1999 Oct 12;38(41):13623-34. PMID:10521269
- ↑ Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B, Jornvall H. Short-chain dehydrogenases/reductases (SDR): the 2002 update. Chem Biol Interact. 2003 Feb 1;143-144:247-53. PMID:12604210
- ↑ Rafi S, Novichenok P, Kolappan S, Zhang X, Stratton CF, Rawat R, Kisker C, Simmerling C, Tonge PJ. Structure of acyl carrier protein bound to FabI, the FASII enoyl reductase from Escherichia coli. J Biol Chem. 2006 Dec 22;281(51):39285-93. Epub 2006 Sep 29. PMID:17012233 doi:10.1074/jbc.M608758200