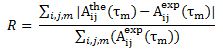RNase A NMR
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:Kroupa RNase Rainbox.png|thumb |400px | + | {{BAMBED |
| - | + | |DATE=May 22, 2011 | |
| + | |OLDID=1247070 | ||
| + | |BAMBEDDOI=10.1002/bmb.20568 | ||
| + | }} | ||
| + | <StructureSection load='2aas' size='350' side='right' scene='Sandbox_Reserved_199/2aas_-_all_models/4' caption='Bovine RNase A NMR structure (PDB code [[2aas]]).'> | ||
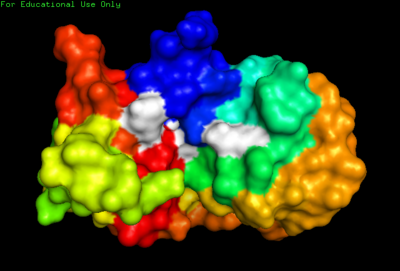
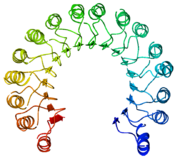
| + | [[Image:Kroupa RNase Rainbox.png|thumb|center |400px |alt=NMR. |2AAS-NMR structure of bovine pancreatic ribonuclease A. Color coded by rainbow gradient typical to NMR sturctures with amino (blue) to carboxy (red). Active site shown in white. ]] | ||
| + | {{Clear}} | ||
== Introduction == | == Introduction == | ||
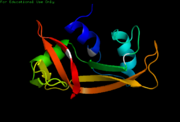
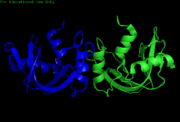
| - | [[Image:Kroupa RNase Cartoon 2.png|thumb |Right|alt=NMR Cartoon. | | + | [[Image:Kroupa RNase Cartoon 2.png|thumb |Right|alt=NMR Cartoon. |[[2aas]]-Cartoon NMR structure of bovine pancreatic Ribonuclease A.]] |
===Overview=== | ===Overview=== | ||
| - | Ribonuclease A has served as a model for protein structure and function, and was the third enzyme whose three-dimensional structure was determined.<ref name="raines"> Raines, Ronald T. "Ribonuclease A." Chemical Reviews; 98, 1045-1065 (1998). Print.</ref> Ribonuclease has been called the most studies enzyme of the 20th century due to its ample availability as well as its significant role within the cell.<ref name="raines"/> While the structures of bovine pancreatic ribonuclease (RNase A) and human pancreatic ribonuclease (RNase 1) determined by X-Ray crystallography have been around for some time, the 3D NMR structures of present provide much more information on specific locations of side chain residues and their flexibility. Because NMR does not require a "frozen" crystal structure, NMR imaging can show much more accurate detail into the actual, solution enzyme (folding, flexibility etc.) | + | '''Ribonuclease A''' has served as a model for protein structure and function, and was the third enzyme whose three-dimensional structure was determined.<ref name="raines"> Raines, Ronald T. "Ribonuclease A." Chemical Reviews; 98, 1045-1065 (1998). Print.</ref> Ribonuclease has been called the most studies enzyme of the 20th century due to its ample availability as well as its significant role within the cell.<ref name="raines"/> While the structures of bovine pancreatic ribonuclease (RNase A) and human pancreatic ribonuclease (RNase 1) determined by X-Ray crystallography have been around for some time, the 3D NMR structures of present provide much more information on specific locations of side chain residues and their flexibility. Because NMR does not require a "frozen" crystal structure, NMR imaging can show much more accurate detail into the actual, solution enzyme (folding, flexibility etc.) |
Ribonuclease A and Ribonuclease 1 are both good targets for 3D NMR. Not only are they small proteins (~13 KDa), they also have numerous characteristics that are observable only by NMR, such as internal flexibility and 3D domain swapping. | Ribonuclease A and Ribonuclease 1 are both good targets for 3D NMR. Not only are they small proteins (~13 KDa), they also have numerous characteristics that are observable only by NMR, such as internal flexibility and 3D domain swapping. | ||
| Line 53: | Line 59: | ||
<scene name='Sandbox_Reserved_199/2aas_-_five_amide_backbone_pro/3'>Five backbone amide protons</scene> were shown to be involved in folding-related intermolecular interactions during initial protein folding steps: amide protons from <scene name='Sandbox_Reserved_199/2aas_-_val_63/2'>Val 63</scene>, <scene name='Sandbox_Reserved_199/2aas_-_ile81/2'>Ile 81</scene>, <scene name='Sandbox_Reserved_199/2aas_-_thr82/1'>Thr 82</scene>, <scene name='Sandbox_Reserved_199/2aas_-_ile106/1'>Ile 106</scene>, and <scene name='Sandbox_Reserved_199/2aas_-_val_118/1'>Val 118</scene>. All five of these protons are involved in hydrogen bonding within the <scene name='Sandbox_Reserved_199/2aas_-_beta_sheet/2'>β sheet secondary structure</scene> of Ribonuclease; therefore, β sheets were proposed to be the starting point for the folding mechanism of Ribonuclease. Furthermore, this supports the formation of a stable secondary structure before the formation of the <scene name='Sandbox_Reserved_199/2aas_-_final_tertiary_structue/1'>final tertiary structure</scene>, which is consistent with the framework model of [http://en.wikipedia.org/wiki/Protein_folding#Protein_nuclear_magnetic_resonance_spectroscopy protein folding mechanisms]. | <scene name='Sandbox_Reserved_199/2aas_-_five_amide_backbone_pro/3'>Five backbone amide protons</scene> were shown to be involved in folding-related intermolecular interactions during initial protein folding steps: amide protons from <scene name='Sandbox_Reserved_199/2aas_-_val_63/2'>Val 63</scene>, <scene name='Sandbox_Reserved_199/2aas_-_ile81/2'>Ile 81</scene>, <scene name='Sandbox_Reserved_199/2aas_-_thr82/1'>Thr 82</scene>, <scene name='Sandbox_Reserved_199/2aas_-_ile106/1'>Ile 106</scene>, and <scene name='Sandbox_Reserved_199/2aas_-_val_118/1'>Val 118</scene>. All five of these protons are involved in hydrogen bonding within the <scene name='Sandbox_Reserved_199/2aas_-_beta_sheet/2'>β sheet secondary structure</scene> of Ribonuclease; therefore, β sheets were proposed to be the starting point for the folding mechanism of Ribonuclease. Furthermore, this supports the formation of a stable secondary structure before the formation of the <scene name='Sandbox_Reserved_199/2aas_-_final_tertiary_structue/1'>final tertiary structure</scene>, which is consistent with the framework model of [http://en.wikipedia.org/wiki/Protein_folding#Protein_nuclear_magnetic_resonance_spectroscopy protein folding mechanisms]. | ||
| - | <Structure load='2AAS' size='350' frame='true' align='right' caption='2AAS - NMR Scructure of Bovine Pancreatic Ribonuclease, [[2aas]]' scene='Sandbox_Reserved_199/2aas_-_all_models/4' /> | ||
==Ribonuclease NMR Structure Versus X-Ray Crystallography Ribonuclease Structure<ref name="Santoro">PMID: 8381876 </ref>== | ==Ribonuclease NMR Structure Versus X-Ray Crystallography Ribonuclease Structure<ref name="Santoro">PMID: 8381876 </ref>== | ||
| Line 78: | Line 83: | ||
==Solution Structure and Dynamics of Human Pancreatic Ribonuclease<ref name="rico">PMID: 18495155</ref>== | ==Solution Structure and Dynamics of Human Pancreatic Ribonuclease<ref name="rico">PMID: 18495155</ref>== | ||
| - | < | + | <scene name='Sandbox_Reserved_199/2k11_all_models/2'>NMR Structure of Human Pancreatic Ribonuclese</scene> ([[2k11]]). |
===Experimental Procedure=== | ===Experimental Procedure=== | ||
| Line 108: | Line 113: | ||
* [http://www.proteopedia.org/wiki/index.php/RNaseS_RNaseB RNase S and RNase B] | * [http://www.proteopedia.org/wiki/index.php/RNaseS_RNaseB RNase S and RNase B] | ||
* [http://www.proteopedia.org/wiki/index.php/RNaseA_Nobel_Prizes RNase A Nobel Prizes] | * [http://www.proteopedia.org/wiki/index.php/RNaseA_Nobel_Prizes RNase A Nobel Prizes] | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
==3D structures of ribonuclease== | ==3D structures of ribonuclease== | ||
| Line 132: | Line 139: | ||
*[http://www.proteopedia.org/wiki/index.php/User:Benjamin_Trefilek Ben Trefilek] | *[http://www.proteopedia.org/wiki/index.php/User:Benjamin_Trefilek Ben Trefilek] | ||
| + | |||
| + | [[Category:Featured in BAMBED]] | ||
Current revision
This page, as it appeared on May 22, 2011, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
3D structures of ribonuclease
References
- ↑ 1.0 1.1 Raines, Ronald T. "Ribonuclease A." Chemical Reviews; 98, 1045-1065 (1998). Print.
- ↑ Dahl, Jeff. X-ray diffraction pattern of crystallized 3Clpro, a SARS protease. (2.1 Angstrom resolution). 2006. Web. 1 Apr. 2011.
- ↑ English Wikipedia. COSY NMR spectrum progesterone. Web. 1 Apr. 2011.
- ↑ Saunders, Martin, Arnold Wishnia, and John G. Kirkwood. "The Nuclear Magnetic Resonance Spectrum of Ribonuclease." Communications to the Editor 79; 20 May (1957). Print.
- ↑ 5.0 5.1 Udgaonkar JB, Baldwin RL. NMR evidence for an early framework intermediate on the folding pathway of ribonuclease A. Nature. 1988 Oct 20;335(6192):694-9. PMID:2845278 doi:http://dx.doi.org/10.1038/335694a0
- ↑ 6.0 6.1 Santoro J, Gonzalez C, Bruix M, Neira JL, Nieto JL, Herranz J, Rico M. High-resolution three-dimensional structure of ribonuclease A in solution by nuclear magnetic resonance spectroscopy. J Mol Biol. 1993 Feb 5;229(3):722-34. PMID:8381876 doi:http://dx.doi.org/10.1006/jmbi.1993.1075
- ↑ 7.0 7.1 Kover KE, Bruix M, Santoro J, Batta G, Laurents DV, Rico M. The solution structure and dynamics of human pancreatic ribonuclease determined by NMR spectroscopy provide insight into its remarkable biological activities and inhibition. J Mol Biol. 2008 Jun 20;379(5):953-65. Epub 2008 Apr 25. PMID:18495155 doi:10.1016/j.jmb.2008.04.042
- ↑ Willow. Top view of ribbon diagram of ribonuclease inhibitor (PDB accession code 2BNH). Made with MOLMOL. 2006. Web. 1 Apr. 2011..
External Resources
Student Contributors
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Angel Herraez, R. Jeremy Johnson, Alexander Berchansky, Daniel Kroupa