|
|
| (440 intermediate revisions not shown.) |
| Line 3: |
Line 3: |
| | <!-- INSERT YOUR SCENES AND TEXT BELOW THIS LINE --> | | <!-- INSERT YOUR SCENES AND TEXT BELOW THIS LINE --> |
| | | | |
| - | =='''Catechol-O-Methyltransferase'''== | + | =='''Fibroblast Growth Factor Receptor/Ponatinib (4uxq) <ref name="one">PMID: 25465127</ref>'''== |
| | + | by Julie Boshar, Emily Boyle, Nicole Kirby, Cory Thomas, Connor Walsh |
| | | | |
| - | ===Introduction===
| + | [[Student Projects for UMass Chemistry 423 Spring 2016]] |
| - | <Structure load='3bwm' size='500' frame='true' align='right' caption='COMT transfer of methyl group from SAM to catecholamine inactivates catecholamine' scene='Insert optional scene name here' /> | + | <StructureSection load='4uxq' size='350' side='right' caption='The Ponatinib-FGFR complex is highly effective for treating CML ([[4uxq]])' scene=''> |
| | | | |
| - | Catechol-O-Methyltransferase (COMT) is an enzyme, which can be either soluble or membrane-bound, that is responsible for the degradation of catecholamine neurotransmitters <ref>PMID:16618795</ref>. This inactivation is accomplished by transferring a <font color='red'>methyl group</font> from <font color='green'>S-adenosyl methionine (SAM)</font> to the <font color='blue'>catecholamine</font>, seen <scene name='Sandbox_Reserved_425/Sam-catecholamine_interaction/9'>here</scene><ref>Grossman, MH, Emanuel, BS, Budarf, ML. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1----q11.2. (1992). Genomics, 12(4), 822-825.</ref>.
| + | ==Introduction== |
| | | | |
| - | One neurotransmitter in this catecholamine family targeted by COMT is dopamine, the neurotransmitter most closely associated with Parkinson's disease. Parkinson's Disease arises out of a lack of dopamine and is characterized by uncontrollable tremors, muscular rigidity, postural instability. At a functional synapse, the action potential prompts release of neurotransmitters like dopamine at the synapse. These neurotransmitters bind to receptors on the postsynaptic membrane, perpetuating the signal. Once the signal has been transmitted, the neurotransmitters are removed from the synapse via reuptake or degradation by enzymes such as COMT. In a person with Parkinson's Disease, dopamine levels are often too low to adequately continue the message to the next neuron. The disease is currently treated with L-DOPA, a dopamine precursor that is converted to dopamine within the brain. However the bioavailability and stability of L-DOPA when used alone is limited. COMT is being investigated as a target for therapeutic agents that would increase the efficacy of L-DOPA. Inhibition of COMT would prevent inactivation of dopamine, leaving higher levels of active dopamine at the synapse and increasing the likelihood of perpetuation of the message to the postsynaptic neuron<ref>Espinoza, S, Manago, F, Leo, D, Sotnikova, TD, Gainetdinov, RR. Role of catechol-O-methyltransferase (COMT)-dependent processes in Parkinson's Disease and L-DOPA treatment. (2012). CNS Neurological Disorder Drug Targets.</ref>.
| + | Potatinib was developed as a treatment option for chronic myeloid leukemia (CML) as other inhibitors in treatment have become ineffective. BCR-ABL is a kinase with a cancerous genetic mutation in chromosome 22 that leaves it always active. Further mutations in BCR-ABL have left earlier drugs that inhibit tyrosine kinases unable to bind in almost 30% of cases after five years of treatment. The newer, mutant BCR-ABL kinase’s ability to develop new resistances has pushed for newer developments in inhibitors, such as Potatinib<ref name="seven">PMID: 21118377 </ref>. |
| | | | |
| - | ===Overall Structure=== | + | Fibroblast growth factor (FGFR) signaling is the factor that normally activates the BCR-ABL kinase. Also, it is the protein behind both tissue development and repair, the disruption of FGFR leads to tumor growth. The activation of BCR-ABL happens through a series of cascading signals that induce proliferation and migration in cells. Mutations in the regulation of the FGFR tyrosine kinases can be diresctly correlated to malignant tumor growth<ref name="one" />. The tyrosine kinase inhibitor Ponatinib has been used to <scene name='48/483882/Activation_loop/1'>bind</scene> to the mutant version of kinase BCR-ABL by the enzyme's specific "DFG-out" conformation (in <font color='turquoise'><b>turquoise</b></font>). This conformation has the phenylalanine group of BCR-ABL flipped out of its hydrophobic binding site. Ponatinib is the first of its kind to be able to inhibit this specific mutation in BCR-ABL of the "DGF-out" conformation<ref name="seven">PMID: 21118377 </ref>. |
| - | <Structure load='2zvj' size='500' frame='true' align='right' caption='The structural differences between Catechol-O-Methyltransferase with a coumarine inhibitor, pdb code 2zvj, compared to a carecholic inhibitor, pdb code 2cl5. ' scene='Insert optional scene name here' /> | + | |
| | | | |
| - | The Catechol-O-Methyltransferase complex, with a coumarine inhibitor, is a monomer that is made up of <font color='deepskyblue'>eight</font> <scene name='Sandbox_Reserved_425/Beta_sheets/1'>Beta Sheets</scene>. These sheets are all parallel except for one. The monomer is also comprised of <scene name='Sandbox_Reserved_425/Alpha_helices/1'>eight</scene> <font color='deepskyblue'>alpha helices</font>. The beta sheets are on the inside of the complex where the alpha helices are on the outside and enclose the beta strands. The Catechol-O-Methyltransferase can either be membrane bound or soluble. Because it can be either, the <font color='magenta'>polar</font> and '''<font color='darkgray'>nonpolar</font>''' residues are seen <scene name='Sandbox_Reserved_425/Polor_and_nonpolar/1'>here</scene>.<ref>PMID:19056347</ref>
| + | Ponatinib's harmful side effects have caused it to fall under scrutiny from the U.S. Food and Drug Administration (FDA). It has shown to increase chances of deadly blood clotting and restenosis in both arteries and veins with a rate of about 1 in 5 patients. The drug has also shown to increase risk of heart attack and overall worsening of heart disease in patients<ref name="seven" />. |
| | | | |
| - | Catechol-O-Methyl Transferase in complex with a catcholic inhibitor which is a '''non-coumarine inihibitor''' can normally form a <scene name='Sandbox_Reserved_425/Dimer_2cl5/1'>dimer</scene> (pdb code 2cl5). For previous inhibitors that have been used and studied this is true but not for the coumarine inhibitor. This dimer can be formed because the ligand that is attached, which in this case in a bacterial inhibitor, BIE, allows the complex to be structurally flexible. The dimer that forms creates a <scene name='Sandbox_Reserved_425/Dimer_pocket/1'>Pocket</scene>, the <font color='deepskyblue'>ligands</font> are enclosed in the center so that the two monomers fit together nicely and have flexibility. As you can see the two ligands slide pass each other and the two <scene name='Sandbox_Reserved_425/Base_stacking/1'>aromatic</scene> rings look as if they are stacked on top of each other. In this pocket the <font color='blue'>S-Adenosyl Methionine</font>, <scene name='Sandbox_Reserved_425/Distance/1'>SAM</scene>, donates a methyl group to each of the ligands present that have the <font color='lime'>magnesium ion</font> attached. The distance between the <font color='blue'>SAM</font> molecules and the ligands are almost exactly equal distances apart from each other creating an even spacing pocket to form making the dimer very symmetric.<ref>PMID:16618795</ref> | |
| | | | |
| - | The complex with the coumarine ligand, pdb code 2zvj, does not allow for such a convenient <scene name='Sandbox_Reserved_425/Monomer_sam_methyl_transfer/1'>distance</scene> between the SAM molecule and ligand to form when they are bound together. Also, with a coumarine inhibitor, the aromatic ring stacking that we saw with the non-coumarine inhibitor does not occur. This asymmetric structure has effects on the binding interactions and therefore the function of the complex.<ref>PMID:19056347</ref>
| + | ==Overall Structure== |
| | + | In terms of <scene name='48/483882/Secondary_structure/2'>secondary structure</scene>, FGFR in complex with Ponatinib consists of two domains, which is the characteristic structure exhibited by kinases. The N-terminal domain is the smaller of the two, and it contains a five-stranded beta sheet and an alpha carbon helix. The larger C-terminal domain is primarily alpha helical in structure. The alpha helices are shown in <font color='fuchsia'><b>fuchsia</b></font> and the beta strands are shown in <font color='orange'><b>orange</b></font>. A hinge links the two regions. A network of hydrogen-bonds between three conserved residues – Glu551, Asn535, and Lys627 – exists in the hinge region. This hydrogen-bonding controls the kinase activity of FGFR. |
| | | | |
| | + | In its active form, FGFR is dimerized and contains two activated intracellular substrates. The binding of a coreceptor, β-Klotho, stabilizes the activated complex. A DFG moiety is found in BCR-ABL, the conformation of which plays a key role in binding Ponatinib. Another defining feature of active FGFR is its <scene name='48/483882/Hydrophobic_spine/1'>hydrophobic spine</scene>. Four residues in the spine – Leu536, Met524, His610, and Phe631 (in <font color='orange'><b>orange</b></font>) – are highly conserved. A gatekeeper residue is present at the beginning of the hinge, and interactions among the four hydrophobic spine residues link the gatekeeper to Tyr643 in the activation loop. This activation loop is glycine-rich and found in the kinase domain of FGFR<ref name="one" />. |
| | | | |
| | + | The structure of Ponatinib is shown as follows: |
| | + | [[Image:StructureP.PNG]] |
| | | | |
| - | ===Binding Interactions=== | |
| - | <Structure load='2zvj' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | |
| - | <font color='cyan'>Met 40</font>, <font color='magenta'>Leu 198</font>, and <font color='midnightblue'>Tyr 200</font> define the <scene name='Sandbox_Reserved_425/Pocket/3'>pocket</scene> for the 4-phenyl-7, 8-dihydroxycoumarine (<font color='dimgray'>4PCM</font>) ligand binding site. <font color='gold'>Trp 38</font> and <font color='darkorange'>Pro 174</font> make <scene name='Sandbox_Reserved_425/Vanderwaals/5'>Van der Waals</scene> interactions with the <font color='dimgray'>4PCM</font>. This also allows us to see how, unlike the ligand described in the Overall Structure section, <font color='dimgray'>4PCM</font> is sterically constrained and unable to form necessary interactions for a dimer configuration. The <font color='lime'>magnesium ion</font> interacts with the two <scene name='Sandbox_Reserved_425/Hydroxyl_groups/1'>hydroxyl groups</scene> of the <font color='dimgray'>4PCM</font>. The <font color='lime'>magnesium ion</font> also aids in the <scene name='Sandbox_Reserved_425/Lys_144/2'>protonation</scene> of <font color='violet'>Lys 144</font>, causing an electrostatic interaction with a hydroxyl group of <font color='dimgray'>4PCM</font>. The other end of this <font color='violet'>Lys 144</font> then acts a hydrogen bond donor for a <font color='darkblue'>water</font> molecule in the binding pocket. This <font color='darkblue'>water</font> molecule then acts a hydrogen bond donor for the carbonyl group of <font color='dimgray'>4PCM</font>, creating an interesting network of hydrogen bonds. The above interactions stabilize the ligand in the binding pocket. These interactions are somewhat similar to other inhibitors previously used, however some differences do occur that make past inhibitors more stable.<ref>PMID:16618795</ref> In our <font color='dimgray'>4PCM</font>, we can see a <scene name='Sandbox_Reserved_425/Met_40/2'>dihedral angle</scene> between the sulfur and carbon atoms of <font color='cyan'>Met 40</font> to be significantly less than <scene name='Sandbox_Reserved_425/3_5-dinitrocatchetol/4'>that</scene> of the <font color='cyan'>Met 40</font> in a COMT complex using <font color='darkred'>3,5-dinitrocatchetol</font><ref>PMID:8127373</ref> as an inhibitor instead; the change is about 96 degrees. This interaction disrupts the favorable binding stabilization interactions of <font color='dimgray'>4PCM</font> with COMT. This interaction, as well as the constraining effects of <font color='gold'>Trp 38</font> and <font color='darkorange'>Pro 174</font> interactions, make <font color='dimgray'>4PCM</font> less stable than nitrocatchetol inhibitors currently being used to treat PD. However, nitrocatchetol inhibitors act as uncouplers, making <font color='dimgray'>4PCM</font> side effects less complex and more attractive. | |
| | | | |
| | + | ==Binding Interactions== |
| | | | |
| | + | Kinases are the largest drug targets being tested. All kinases possess a biolobal fold that connects the N and C termini by a “hinge” that binds ATP. “Gatekeepers” are other residues that are in the hinge and alter binding capabilities. Mutations to gatekeepers are critical considerations in drug development because they can result in drug resistances<ref name="four">PMID: 25317566</ref>. Ponatinib can bind even in the presence of gatekeeper mutations, like T315I which accounts for 15-20% of all clinically observed mutations and is resistant to all previous generation drugs<ref name="five">PMID: 25219510</ref>. This class of inhibitors can bind deep within the hydrophobic and allosteric pocket that is only accessible in the <scene name='48/483882/Active_sitezoom/1'>DFG-out</scene> conformation (see color chart) which consists of Asp630, Phe631, and Glu632<ref name="four" />. |
| | | | |
| - | ===Additional Features===
| + | <center><big>{{Template:ColorKey_N52C3Rainbow}}.</big></center> |
| | | | |
| - | <Structure load='2zvj' size='500' frame='true' align='right' caption='The location at position 158 where valine replaces with methionine (Val148Met) in the COMT enzyme activity' scene='Insert optional scene name here'/> | + | Ponatinib binds to the ATP binding pocket between the N and C lobes to shift from the DFG-in to the DFG-out conformation. It spans from the <scene name='48/483882/Hinge/1'>hinge</scene> (<font color='cyan'><b>cyan</b></font>) to the front catalytic pocket. Three sites are engaged in the ATP binding cleft by ponatinib’s aromatic rings. First, imidazo[1,2b]pyridazine occupies the same space as the adenine ring of ATP and forms a <scene name='48/483882/Hingehbond/1'>hydrogen bond</scene> with the amide nitrogen atom of Ala553 (<font color='magenta'><b>magenta</b></font>) in the hinge<ref name="seven" />. Multiple triple bonds help the rest of ponatinib to move further in to the ATP binding pocket. Second, the methylphenyl group displaces the side chain of Lys503 and its aromatic ring binds to the hydrophobic pocket that is formed by Val550 (the <scene name='48/483882/Gatekeeper/1'>gatekeeper</scene> in <font color='yellow'><b>yellow</b></font>), and Met524. This allows Glu520 to hydrogen bond with the amide linkage between the aromatic rings in ponatinib. Third, Phe631 is replaced by ponatinib’s 3-trifluoromethylphenyl group. Asp630 becomes available for hydrogen bonding with the amide linkage between ponatinib’s aromatic rings and lets the piperazine ring hydrogen bond with the catalytic loop which forms the DFG-out conformation<ref name="seven" />. |
| | | | |
| - | In the gene for COMT, there is a functional single-nucleotide polymorphism that switches from valine to methionine mutation at position 158 (Va <scene name='Sandbox_Reserved_425/158/1'>158</scene>Met). The Val variations can breakdowns dopamine as high as four times the rate as its methionine. As a result, neurotransmitter is then release due to the lower dopamine levels. Since COMT's role is to degraded dopamine, the Val158Met polymorphism is told to utilize its effects on cognition by regulating dopamine signaling in the front area of the human brain. <ref>Rakvåg TT, Klepstad P, Baar C, Kvam TM, Dale O, Kaasa S, Krokan HE, Skorpen F. "Molecular Pain | Full Text | Genetic Variation in the Catechol-O-Methyltransferase (COMT) Gene and Morphine Requirements in Cancer Patients with Pain." Molecular Pain. Web. 25 Apr. 2012. <http://www.molecularpain.com/content/4/1/64>.</ref> There are drugs in the market that could focus only in the frontal lobes and prevent Val158Met to happen. The two common <font color='blue'>major</font> inhibitor drugs (<font color='darkorchid'>tolcapone </font>and <font color='green'>entacapone</font>) that are sold on the market, inhibit the action of COMT. These drugs effectively reduce / inhibit COMT’s ability to degrade neurotransmitters. As mentioned earlier, these drugs are mainly used to combat Parkinson’s Disease.
| + | ==Additional Features== |
| | | | |
| - | <font color='darkorchid'>Tolcapone </font>, which inhibits COMT from immediately converting L-DOPA into 3-O-methyldopa, has the ability to facilitate higher levels of L-DOPA conversion in the central nervous system. Additionally, it allows dopamine to hang around longer by preventing its degradation. Unfortunately, this has also lead to the discovery that Tolcapone promotes high levels of hepatotoxicity (chemical-driven liver damage). This negative side effect has limited the usage of Tolcapone and promoted the selection of another inhibitor drug: Entacapone. <ref>"Why is this medication prescribed?" Entacapone. 18 Dec. -0001. U.S. National Library of Medicine. 25 Apr. 2012 <http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000168/>.</ref>
| + | Ponatinib is an orally ingested tyrosine kinase inhibitor that has revealed successful avenues of treatment for counteracting the effects of angiogenesis in tumor growth. Besides the inhibition of FGFRs, this agent inhibits tyrosine kinases involved in vascular endothelial growth factor receptors. Ponatinib is considered a third generation TKI that can treat even the most drug-therapy resistant mutations that previous TKIs were incapable of treating<ref name="eight">PMID: 23986642</ref>. |
| | | | |
| - | <font color='green'>Entacapone</font>, unlike Tolcapone, does not easily cross the blood-brain barrier. As a result, it does not cause hepatotoxicity, and is therefore a very common choice. It is typically used to treat the “end-of-dose ‘wearing-off’ symptoms of Parkinson’s Disease. Entacapone is typically used with Levodopa and its role is to prevent the breakdown of L-DOPA OUTSIDE of the brain. Recall that L-DOPA can be broken down into Dopamine, but too much Dopamine throughout the body could be harmful. As a result, Entacapone restores normal cognitive function in patients but also limits the side effects of dopamine on the rest of the body. <ref>"Why is this medication prescribed?" Entacapone. 18 Dec. -0001. U.S. National Library of Medicine. 25 Apr. 2012 <http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000168/>.</ref>
| + | The brand name for ponatinib is Iclusig. Iclusig received an accelerated approval grant through the Food and Drug Administration. It was mainly prescribed to patients suffering from Chronic Myeloid Leukemia or Acute Lymphoblastic Leukemia who did not make any progress with the first and second generation TKIs. However, the clinical trials data displayed a spike in adverse effects. These consequences include heart failure, stroke, coronary artery disease, loss of blood flow to body parts leading to amputation amongst other narrowing of blood vessels<ref>FDA Drug Safety Communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins; Drug Safety and Availability; United States Food and Drug Administration (2013). Web. [http://www.fda.gov/Drugs/DrugSafety/ucm370945.htm]</ref>. |
| | | | |
| - | ===Credits=== | + | FGFR-4 is abundantly present in human prostate cancer observed in vitro and in mouse model simulations<ref name="nine">PMID: 22573348</ref>. A <scene name='48/483882/Variant/9'>variant</scene> of FGFR-4 with Arg388 replacing Gly388 is implicated with increased human prostate cancer. This variation causes increased receptor stability and activation<ref name="ten">PMID:18670643</ref>. A study revealed that the <scene name='48/483882/Inhibition/1'>inhibition</scene> of FGFR-4 signaling completely curtailed prostate cancer cell lines that were responsible for tumor growth<ref name="nine">PMID: 22573348</ref>. Due to the significant results of diminished cell growth in treated tumors, targeting fibroblast growth factor signaling appears to provide a promising step towards combating aggressive prostate cancer. |
| | | | |
| - | Introduction - Jessica Royal | |
| | | | |
| - | Overall Structure - Stephanie Bristol | |
| | | | |
| - | Drug Binding Site - Emily Brackett
| + | ==Quiz Question 1== |
| | | | |
| - | Additional Features - Anh Huynh
| + | Ponatinib is unique in it's ability to bind to the mutated BCR-ABL because of it's preference to shift to the DFG-out conformation. In theory, if a competitive inhibitor was created by nature to prevent Ponatinib from binding to BCR-ABL to further its drug resistance, what specific structural characteristics would the inhibitor need to possess? Consider the unique binding methods of Ponatinib and the <scene name='48/483882/Active_sitezoom/1'>DFG-out</scene> conformation. |
| | + | |
| | + | a. Small, fully conjugated aromatic system with no electronegative substituents, to prevent unwanted hydrogen bonding. |
| | | | |
| - | ===References=== | + | b. Multiple ring system, one ring particularly for hydrogen bonding and another capable of binding in a hydrophobic pocket. |
| | + | |
| | + | c. Polymer chain with an ester linkage and a hydroxyl end group . |
| | + | |
| | + | d. Metal center that binds four large, nonpolar hydrocarbon ligands that exhibit significant steric hindrance. |
| | + | |
| | + | ==See Also== |
| | + | *[[Fibroblast growth factor receptor]] |
| | + | *[[4uxq]] |
| | + | *[[4v01]] |
| | + | *[[4v04]] |
| | + | *[[4v05]] |
| | + | |
| | + | ==Credits== |
| | + | |
| | + | Introduction - Emily & Cory* |
| | + | |
| | + | Overall Structure - Nicole* & Connor |
| | + | |
| | + | Drug Binding Site - Julie* & Cory |
| | + | |
| | + | Additional Features - Connor* & Nicole |
| | + | |
| | + | Quiz Question 1 - Julie & Emily* |
| | + | |
| | + | ==References== |
| | <references/> | | <references/> |
|
Introduction
Potatinib was developed as a treatment option for chronic myeloid leukemia (CML) as other inhibitors in treatment have become ineffective. BCR-ABL is a kinase with a cancerous genetic mutation in chromosome 22 that leaves it always active. Further mutations in BCR-ABL have left earlier drugs that inhibit tyrosine kinases unable to bind in almost 30% of cases after five years of treatment. The newer, mutant BCR-ABL kinase’s ability to develop new resistances has pushed for newer developments in inhibitors, such as Potatinib[2].
Fibroblast growth factor (FGFR) signaling is the factor that normally activates the BCR-ABL kinase. Also, it is the protein behind both tissue development and repair, the disruption of FGFR leads to tumor growth. The activation of BCR-ABL happens through a series of cascading signals that induce proliferation and migration in cells. Mutations in the regulation of the FGFR tyrosine kinases can be diresctly correlated to malignant tumor growth[1]. The tyrosine kinase inhibitor Ponatinib has been used to to the mutant version of kinase BCR-ABL by the enzyme's specific "DFG-out" conformation (in turquoise). This conformation has the phenylalanine group of BCR-ABL flipped out of its hydrophobic binding site. Ponatinib is the first of its kind to be able to inhibit this specific mutation in BCR-ABL of the "DGF-out" conformation[2].
Ponatinib's harmful side effects have caused it to fall under scrutiny from the U.S. Food and Drug Administration (FDA). It has shown to increase chances of deadly blood clotting and restenosis in both arteries and veins with a rate of about 1 in 5 patients. The drug has also shown to increase risk of heart attack and overall worsening of heart disease in patients[2].
Overall Structure
In terms of , FGFR in complex with Ponatinib consists of two domains, which is the characteristic structure exhibited by kinases. The N-terminal domain is the smaller of the two, and it contains a five-stranded beta sheet and an alpha carbon helix. The larger C-terminal domain is primarily alpha helical in structure. The alpha helices are shown in fuchsia and the beta strands are shown in orange. A hinge links the two regions. A network of hydrogen-bonds between three conserved residues – Glu551, Asn535, and Lys627 – exists in the hinge region. This hydrogen-bonding controls the kinase activity of FGFR.
In its active form, FGFR is dimerized and contains two activated intracellular substrates. The binding of a coreceptor, β-Klotho, stabilizes the activated complex. A DFG moiety is found in BCR-ABL, the conformation of which plays a key role in binding Ponatinib. Another defining feature of active FGFR is its . Four residues in the spine – Leu536, Met524, His610, and Phe631 (in orange) – are highly conserved. A gatekeeper residue is present at the beginning of the hinge, and interactions among the four hydrophobic spine residues link the gatekeeper to Tyr643 in the activation loop. This activation loop is glycine-rich and found in the kinase domain of FGFR[1].
The structure of Ponatinib is shown as follows:
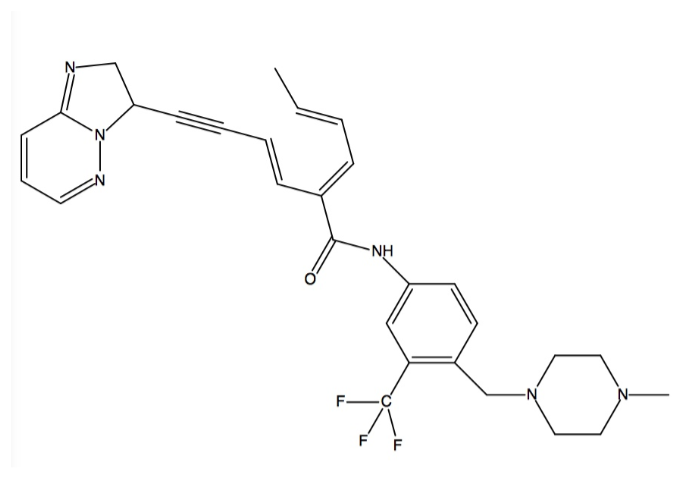
Binding Interactions
Kinases are the largest drug targets being tested. All kinases possess a biolobal fold that connects the N and C termini by a “hinge” that binds ATP. “Gatekeepers” are other residues that are in the hinge and alter binding capabilities. Mutations to gatekeepers are critical considerations in drug development because they can result in drug resistances[3]. Ponatinib can bind even in the presence of gatekeeper mutations, like T315I which accounts for 15-20% of all clinically observed mutations and is resistant to all previous generation drugs[4]. This class of inhibitors can bind deep within the hydrophobic and allosteric pocket that is only accessible in the conformation (see color chart) which consists of Asp630, Phe631, and Glu632[3].
.
Ponatinib binds to the ATP binding pocket between the N and C lobes to shift from the DFG-in to the DFG-out conformation. It spans from the (cyan) to the front catalytic pocket. Three sites are engaged in the ATP binding cleft by ponatinib’s aromatic rings. First, imidazo[1,2b]pyridazine occupies the same space as the adenine ring of ATP and forms a with the amide nitrogen atom of Ala553 (magenta) in the hinge[2]. Multiple triple bonds help the rest of ponatinib to move further in to the ATP binding pocket. Second, the methylphenyl group displaces the side chain of Lys503 and its aromatic ring binds to the hydrophobic pocket that is formed by Val550 (the in yellow), and Met524. This allows Glu520 to hydrogen bond with the amide linkage between the aromatic rings in ponatinib. Third, Phe631 is replaced by ponatinib’s 3-trifluoromethylphenyl group. Asp630 becomes available for hydrogen bonding with the amide linkage between ponatinib’s aromatic rings and lets the piperazine ring hydrogen bond with the catalytic loop which forms the DFG-out conformation[2].
Additional Features
Ponatinib is an orally ingested tyrosine kinase inhibitor that has revealed successful avenues of treatment for counteracting the effects of angiogenesis in tumor growth. Besides the inhibition of FGFRs, this agent inhibits tyrosine kinases involved in vascular endothelial growth factor receptors. Ponatinib is considered a third generation TKI that can treat even the most drug-therapy resistant mutations that previous TKIs were incapable of treating[5].
The brand name for ponatinib is Iclusig. Iclusig received an accelerated approval grant through the Food and Drug Administration. It was mainly prescribed to patients suffering from Chronic Myeloid Leukemia or Acute Lymphoblastic Leukemia who did not make any progress with the first and second generation TKIs. However, the clinical trials data displayed a spike in adverse effects. These consequences include heart failure, stroke, coronary artery disease, loss of blood flow to body parts leading to amputation amongst other narrowing of blood vessels[6].
FGFR-4 is abundantly present in human prostate cancer observed in vitro and in mouse model simulations[7]. A of FGFR-4 with Arg388 replacing Gly388 is implicated with increased human prostate cancer. This variation causes increased receptor stability and activation[8]. A study revealed that the of FGFR-4 signaling completely curtailed prostate cancer cell lines that were responsible for tumor growth[7]. Due to the significant results of diminished cell growth in treated tumors, targeting fibroblast growth factor signaling appears to provide a promising step towards combating aggressive prostate cancer.
Quiz Question 1
Ponatinib is unique in it's ability to bind to the mutated BCR-ABL because of it's preference to shift to the DFG-out conformation. In theory, if a competitive inhibitor was created by nature to prevent Ponatinib from binding to BCR-ABL to further its drug resistance, what specific structural characteristics would the inhibitor need to possess? Consider the unique binding methods of Ponatinib and the conformation.
a. Small, fully conjugated aromatic system with no electronegative substituents, to prevent unwanted hydrogen bonding.
b. Multiple ring system, one ring particularly for hydrogen bonding and another capable of binding in a hydrophobic pocket.
c. Polymer chain with an ester linkage and a hydroxyl end group .
d. Metal center that binds four large, nonpolar hydrocarbon ligands that exhibit significant steric hindrance.
See Also
Credits
Introduction - Emily & Cory*
Overall Structure - Nicole* & Connor
Drug Binding Site - Julie* & Cory
Additional Features - Connor* & Nicole
Quiz Question 1 - Julie & Emily*
References
- ↑ 1.0 1.1 1.2 Tucker JA, Klein T, Breed J, Breeze AL, Overman R, Phillips C, Norman RA. Structural Insights into FGFR Kinase Isoform Selectivity: Diverse Binding Modes of AZD4547 and Ponatinib in Complex with FGFR1 and FGFR4. Structure. 2014 Dec 2;22(12):1764-74. doi: 10.1016/j.str.2014.09.019. Epub 2014, Nov 20. PMID:25465127 doi:http://dx.doi.org/10.1016/j.str.2014.09.019
- ↑ 2.0 2.1 2.2 2.3 2.4 Zhou T, Commodore L, Huang WS, Wang Y, Thomas M, Keats J, Xu Q, Rivera VM, Shakespeare WC, Clackson T, Dalgarno DC, Zhu X. Structural Mechanism of the Pan-BCR-ABL Inhibitor Ponatinib (AP24534): Lessons for Overcoming Kinase Inhibitor Resistance. Chem Biol Drug Des. 2011 Jan;77(1):1-11. doi:, 10.1111/j.1747-0285.2010.01054.x. Epub 2010 Nov 30. PMID:21118377 doi:10.1111/j.1747-0285.2010.01054.x
- ↑ 3.0 3.1 Huang Z, Tan L, Wang H, Liu Y, Blais S, Deng J, Neubert TA, Gray NS, Li X, Mohammadi M. DFG-out Mode of Inhibition by an Irreversible Type-1 Inhibitor Capable of Overcoming Gate-Keeper Mutations in FGF Receptors. ACS Chem Biol. 2014 Oct 27. PMID:25317566 doi:http://dx.doi.org/10.1021/cb500674s
- ↑ Lesca E, Lammens A, Huber R, Augustin M. Structural analysis of the human Fibroblast Growth Factor Receptor 4 Kinase. J Mol Biol. 2014 Sep 11. pii: S0022-2836(14)00474-4. doi:, 10.1016/j.jmb.2014.09.004. PMID:25219510 doi:http://dx.doi.org/10.1016/j.jmb.2014.09.004
- ↑ Price KE, Saleem N, Lee G, Steinberg M. Potential of ponatinib to treat chronic myeloid leukemia and acute lymphoblastic leukemia. Onco Targets Ther. 2013 Aug 20;6:1111-8. doi: 10.2147/OTT.S36980. eCollection, 2013. PMID:23986642 doi:http://dx.doi.org/10.2147/OTT.S36980
- ↑ FDA Drug Safety Communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins; Drug Safety and Availability; United States Food and Drug Administration (2013). Web. [1]
- ↑ 7.0 7.1 Feng S, Shao L, Yu W, Gavine P, Ittmann M. Targeting fibroblast growth factor receptor signaling inhibits prostate cancer progression. Clin Cancer Res. 2012 Jul 15;18(14):3880-8. doi: 10.1158/1078-0432.CCR-11-3214., Epub 2012 May 9. PMID:22573348 doi:http://dx.doi.org/10.1158/1078-0432.CCR-11-3214
- ↑ Wang J, Yu W, Cai Y, Ren C, Ittmann MM. Altered fibroblast growth factor receptor 4 stability promotes prostate cancer progression. Neoplasia. 2008 Aug;10(8):847-56. PMID:18670643
|

