Vinculin
From Proteopedia
(Difference between revisions)
| (26 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
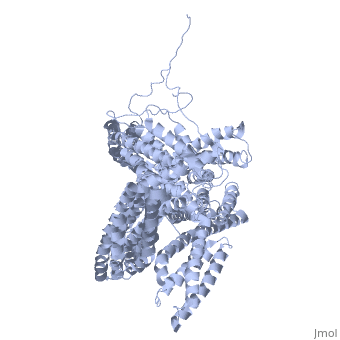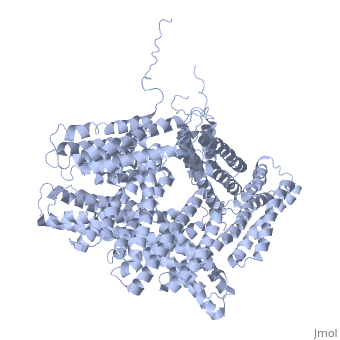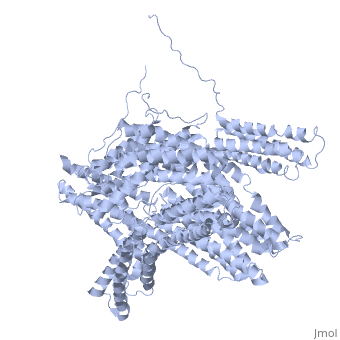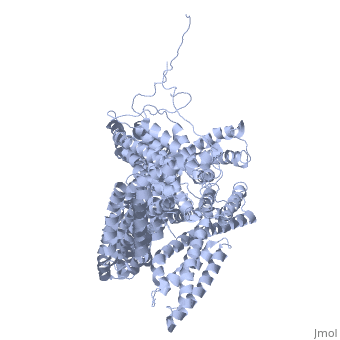
| - | + | <StructureSection load='1st6' size='340' side='right' caption='Chicken full-length metavinculin, [[1st6]]' scene='' > | |
| - | + | == Function == | |
| + | [[Vinculin|Vinculins]] (VCLs) are involved in adhesion by linking integrin molecules to the actin cytoskeleton. Its head domain (Vd1) can bind to [[Talin|talin]] or to [[Actinin|alpha-actinin]] at their respective VCL Binding Sites (VBS)<ref>PMID:11152287</ref>. The protein raver1 RNA Recognition Motif (RRM) forms a complex with VCL or m-VCL. '''Metavinculin''' (m-VCL) is a splice version of VCL containing an extra ca. 70 amino acids in the C-terminal domain. | ||
| - | + | == Relevance == | |
| + | Loss of VCL could be used as a prognostic factor for colorectal cancer se it promotes metastasis<ref>PMID:25496021</ref>. | ||
| + | == Disease == | ||
| + | Mutation in m-VCL can yield cardiomyopathic phenotype<ref>PMID:16236538</ref>. | ||
| + | |||
| + | == Structural highlights == | ||
| + | <scene name='Sandbox_27/Role_i997_vinculin_head-tail_1/1'>Vinculin Autoinhibition</scene> is achieved through a high affinity intramolecular interaction between tail (orange) and head (aqua) domains ([[1st6]]). Energetically, I997 is key to maintaining this autoinhibition. | ||
== 3D Structures of Vinculin == | == 3D Structures of Vinculin == | ||
| + | [[Vinculin 3D structures]] | ||
| - | + | ==References== | |
| + | <references /> | ||
| + | </StructureSection> | ||
| + | [[Category:Topic Page]] | ||
| - | + | *Created with the participation of [[User:Susan Craig|Susan Craig]]. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | Created with the participation of [[User:Susan Craig|Susan Craig]]. | + | |
Current revision
| |||||||||||
- Created with the participation of Susan Craig.