This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
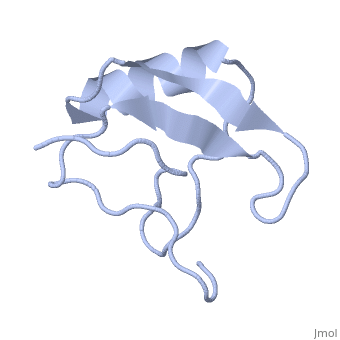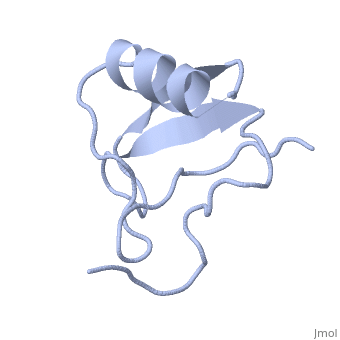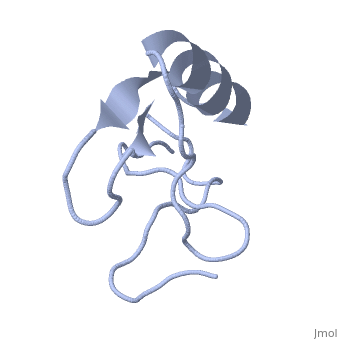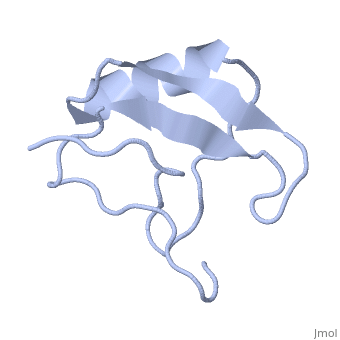
1lqh
From Proteopedia
(Difference between revisions)
m (Protected "1lqh" [edit=sysop:move=sysop]) |
|||
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:1lqh.png|left|200px]] | ||
| - | + | ==INSECTICIDAL ALPHA SCORPION TOXIN ISOLATED FROM THE VENOM OF SCORPION LEIURUS QUINQUESTRIATUS HEBRAEUS, NMR, MINIMIZED AVERAGE STRUCTURE== | |
| - | + | <StructureSection load='1lqh' size='340' side='right'caption='[[1lqh]]' scene=''> | |
| - | + | == Structural highlights == | |
| - | + | <table><tr><td colspan='2'>[[1lqh]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Leiurus_quinquestriatus_hebraeus Leiurus quinquestriatus hebraeus]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1LQH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1LQH FirstGlance]. <br> | |
| - | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> | |
| - | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1lqh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1lqh OCA], [https://pdbe.org/1lqh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1lqh RCSB], [https://www.ebi.ac.uk/pdbsum/1lqh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1lqh ProSAT]</span></td></tr> | |
| - | + | </table> | |
| - | + | == Function == | |
| - | + | [https://www.uniprot.org/uniprot/SCXA_LEIHE SCXA_LEIHE] Alpha toxins bind voltage-independently at site-3 of sodium channels (Nav) and inhibit the inactivation of the activated channels, thereby blocking neuronal transmission. The dissociation is voltage-dependent. This toxin is active on insects. It is also highly toxic to crustaceans and has a measurable but low toxicity to mice. | |
| - | + | == Evolutionary Conservation == | |
| - | + | [[Image:Consurf_key_small.gif|200px|right]] | |
| - | < | + | Check<jmol> |
| - | + | <jmolCheckbox> | |
| - | + | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/lq/1lqh_consurf.spt"</scriptWhenChecked> | |
| - | + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |
| - | + | <text>to colour the structure by Evolutionary Conservation</text> | |
| - | + | </jmolCheckbox> | |
| - | == | + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1lqh ConSurf]. |
| - | [[1lqh]] is a 1 chain structure | + | <div style="clear:both"></div> |
==See Also== | ==See Also== | ||
| - | *[[ | + | *[[Hemolysin 3D structures|Hemolysin 3D structures]] |
| - | + | *[[Potassium channel toxin|Potassium channel toxin]] | |
| - | + | *[[Potassium channel toxin 3D structures|Potassium channel toxin 3D structures]] | |
| - | + | __TOC__ | |
| + | </StructureSection> | ||
| + | [[Category: Large Structures]] | ||
[[Category: Leiurus quinquestriatus hebraeus]] | [[Category: Leiurus quinquestriatus hebraeus]] | ||
| - | [[Category: Anglister | + | [[Category: Anglister J]] |
| - | [[Category: Gurevitz | + | [[Category: Gurevitz M]] |
| - | [[Category: Kustanovich | + | [[Category: Kustanovich I]] |
| - | [[Category: Tugarinov | + | [[Category: Tugarinov V]] |
| - | [[Category: Zilberberg | + | [[Category: Zilberberg N]] |
| - | + | ||
| - | + | ||
Current revision
INSECTICIDAL ALPHA SCORPION TOXIN ISOLATED FROM THE VENOM OF SCORPION LEIURUS QUINQUESTRIATUS HEBRAEUS, NMR, MINIMIZED AVERAGE STRUCTURE
| |||||||||||