Leishmania infantum Glyoxalase II
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
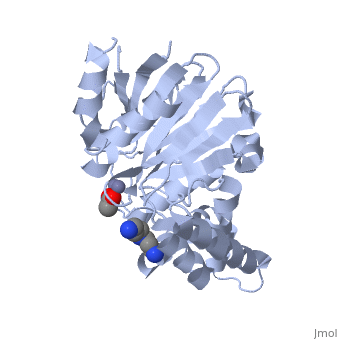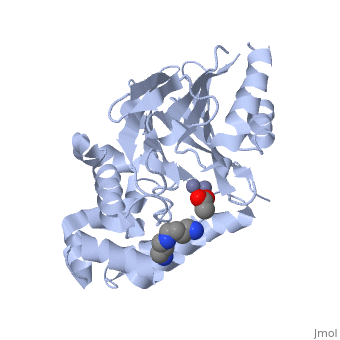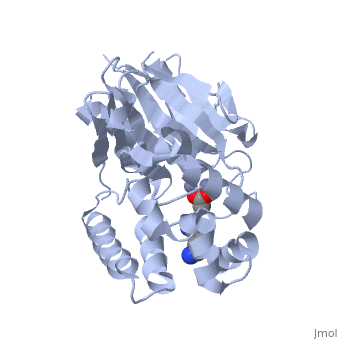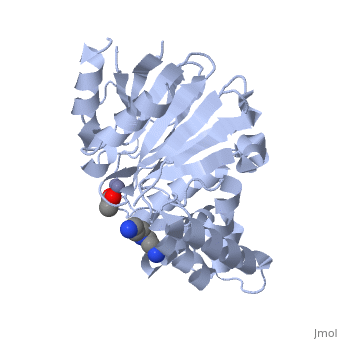
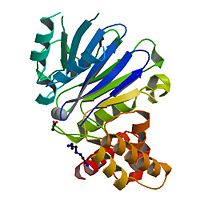
| - | <StructureSection load="2p18" size="400" color="" frame="true" spin="on" Scene= | + | <StructureSection load="2p18" size="400" color="" frame="true" spin="on" Scene= side="right" caption="Glyoxalase II complex with spermidine, acetate and Zn+2 ion, [[2p18]]" > |
[[Image:2p18.jpg|left|200px]] | [[Image:2p18.jpg|left|200px]] | ||
| Line 9: | Line 9: | ||
==Overview== | ==Overview== | ||
| - | The glyoxalase pathway catalyzes the formation of D-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. | + | The '''glyoxalase''' pathway catalyzes the formation of D-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. |
Evolutionary analysis shows that trypanosomatid ''L. infantum'' glyoxalase II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins. This enzyme shows absolute specificity towards trypanothione, making it an exceptional model to understand the molecular basis of trypanothione binding and specificity. | Evolutionary analysis shows that trypanosomatid ''L. infantum'' glyoxalase II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins. This enzyme shows absolute specificity towards trypanothione, making it an exceptional model to understand the molecular basis of trypanothione binding and specificity. | ||
| Line 29: | Line 29: | ||
==The ''L. infantum'' Glyoxalase II Structure== | ==The ''L. infantum'' Glyoxalase II Structure== | ||
| - | The crystal structure of ''Leishmania infantum'' glyoxalase II ( | + | The crystal structure of ''Leishmania infantum'' glyoxalase II ([[2p18]]) is the first structure of this enzyme from trypanosomatids. It is a 295 amino acid metalloprotein. The overall structure of Leishmania infantum glyoxalase II is very similar to its human counterpart. Like the human glx II, it is a monomer arranged in two domains and its active site is very conserved. However it shows important differences at the substrate binding site. |
Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P18 OCA]. | Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P18 OCA]. | ||
| + | |||
| + | ==3D structure of glyoxalase== | ||
| + | |||
| + | [[Glyoxalase]] | ||
| + | |||
==Reference== | ==Reference== | ||
Current revision
| |||||||||||