Bovine odorant binding protein
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='1OBP' size='350' side='right' scene='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Homodimer/1' caption='Bovine odorant-binding protein (PDB code [[1obp]])'> | ||
'''''Bovine Odorant Binding Protein''''' | '''''Bovine Odorant Binding Protein''''' | ||
---- | ---- | ||
| - | [[Image:1GT1.jpg|300px| | + | [[Image:1GT1.jpg|300px|left|thumb| Crystal structure of Bovine Odorant Binding Protein]] [[Image:cow.jpg|300px|left|thumb| Cow]] |
| + | {{Clear}} | ||
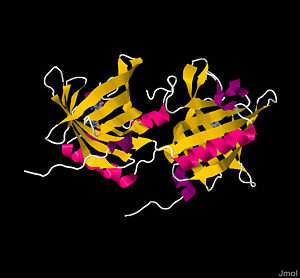
Imagine a world without smells: no fresh cookies, no Valentine’s Day roses, and no stinky gym socks. The sense of smell clearly plays a unique role in how humans and other organisms experience their environment. Unlike the physical objects we see and touch, scents travel invisibly through the air, enter our nose, and elicit a sensory perception. The transduction for this sensory mechanism is crucial in eliciting neuronal activity to stimulate the brain and register that you "stopped and smelled the roses." Before transduction starts, however, select smell molecules, called odorants, must cross a water mucosa layer to reach an extracellular receptor. In bovines, this feat is performed by '''Bovine Odorant Binding Protein (bOBP)''', a small homodimeric protein composed of two β-barrels that utilize a hydrophobic ‘trap’ to transport and release odorants at cellular receptors. | Imagine a world without smells: no fresh cookies, no Valentine’s Day roses, and no stinky gym socks. The sense of smell clearly plays a unique role in how humans and other organisms experience their environment. Unlike the physical objects we see and touch, scents travel invisibly through the air, enter our nose, and elicit a sensory perception. The transduction for this sensory mechanism is crucial in eliciting neuronal activity to stimulate the brain and register that you "stopped and smelled the roses." Before transduction starts, however, select smell molecules, called odorants, must cross a water mucosa layer to reach an extracellular receptor. In bovines, this feat is performed by '''Bovine Odorant Binding Protein (bOBP)''', a small homodimeric protein composed of two β-barrels that utilize a hydrophobic ‘trap’ to transport and release odorants at cellular receptors. | ||
| Line 21: | Line 23: | ||
=='''Structure'''== | =='''Structure'''== | ||
| - | |||
| - | <Structure load='1OBP' size='400' frame='true' align='right' | CAPTION="Bovine odorant-binding protein [[1obp]]" | scene='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Homodimer/1'/> | ||
| - | |||
===''Basic Structural Parameters''=== | ===''Basic Structural Parameters''=== | ||
bOBP is a 159 residue <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Homodimer/1'>homodimer</scene>, with monomers respectively labeled blue and green, of noncovalently bound, virtually identical monomeric subunits<ref name="one" /><ref name="five">PMID: 8901871</ref>. Monomers are synthesized by cell independently, but their assembly process is not well understood. The most significant difference between the two monomers arises at the <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/C-termini/2'>C-termini</scene>; the terminal amino acids 151-159, shown in red, form a short α-helix in one subunit and a disordered tail in the other. Although electron densities at the N-termini of each subunit also differ, the underlying structural chemistry is currently unexplained<ref name="four" />. Other than the aforementioned minor differences, subunits are identical. The total protein itself is small, weighing a mere 19 kDa<ref name="one" />. Each monomeric subunit consists of eight <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Monomer/1'> eight β-sheets, followed by an α-helix, ending in one final β-sheet</scene><ref name="one" /><ref name="four" /><ref name="five" />, with the illustration showing the protein in a progressive reverse [http://en.wikipedia.org/wiki/Color_spectrum color spectrum] (BGYOR), with the N-terminus as blue and the C-terminus as red. The β-sheets loop tightly to form a [http://en.wikipedia.org/wiki/Beta_barrel β barrel]. | bOBP is a 159 residue <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Homodimer/1'>homodimer</scene>, with monomers respectively labeled blue and green, of noncovalently bound, virtually identical monomeric subunits<ref name="one" /><ref name="five">PMID: 8901871</ref>. Monomers are synthesized by cell independently, but their assembly process is not well understood. The most significant difference between the two monomers arises at the <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/C-termini/2'>C-termini</scene>; the terminal amino acids 151-159, shown in red, form a short α-helix in one subunit and a disordered tail in the other. Although electron densities at the N-termini of each subunit also differ, the underlying structural chemistry is currently unexplained<ref name="four" />. Other than the aforementioned minor differences, subunits are identical. The total protein itself is small, weighing a mere 19 kDa<ref name="one" />. Each monomeric subunit consists of eight <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Monomer/1'> eight β-sheets, followed by an α-helix, ending in one final β-sheet</scene><ref name="one" /><ref name="four" /><ref name="five" />, with the illustration showing the protein in a progressive reverse [http://en.wikipedia.org/wiki/Color_spectrum color spectrum] (BGYOR), with the N-terminus as blue and the C-terminus as red. The β-sheets loop tightly to form a [http://en.wikipedia.org/wiki/Beta_barrel β barrel]. | ||
| Line 50: | Line 49: | ||
=='''Functions'''== | =='''Functions'''== | ||
| - | < | + | <scene name='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Ligand/1'>Natural Ligand of bOBP</scene> |
===''Chemical Function''=== | ===''Chemical Function''=== | ||
| + | |||
| + | |||
The exact function of bOBP is not well defined. Although OBP primarily transports hydrophobic odorants across the hydrophilic nasal mucosa, this may not be its only role. The first alternative role tributes bOBP with regulating the concentrations odorant concentrations through buffering capabilities<ref name="three" />. With high K<sub>d</sub> values, bOBP can trap odorants more efficiently at high concentration and subsequently narrow the wide range of possible stimuli intensities<ref name="one" /><ref name="two" />. A second theory expands on bOBPs transporter role, claiming it may mediate odorant signal transduction by interacting with the odorant receptor as an odorant-OBP complex<ref name="one" />. | The exact function of bOBP is not well defined. Although OBP primarily transports hydrophobic odorants across the hydrophilic nasal mucosa, this may not be its only role. The first alternative role tributes bOBP with regulating the concentrations odorant concentrations through buffering capabilities<ref name="three" />. With high K<sub>d</sub> values, bOBP can trap odorants more efficiently at high concentration and subsequently narrow the wide range of possible stimuli intensities<ref name="one" /><ref name="two" />. A second theory expands on bOBPs transporter role, claiming it may mediate odorant signal transduction by interacting with the odorant receptor as an odorant-OBP complex<ref name="one" />. | ||
| Line 59: | Line 60: | ||
=='''Functional Relatives'''== | =='''Functional Relatives'''== | ||
| - | |||
| - | <Structure load='2hlv' size='300' frame='true' align='left' caption='Mutant bOBP [[2hiv]]' scene='User:Michael_Kerins/Bovine_Odorant_Binding_Protein/Mutant/1' /> | ||
===''Mutants''=== | ===''Mutants''=== | ||
| Line 72: | Line 71: | ||
=='''Summary'''== | =='''Summary'''== | ||
Bovine odorant binding protein is a small homodimeric protein found in the nasal mucosa of cows; it has numerous possible functions, including odorant buffering, cellular transduction, insect repellant, and its most important function: odorant transport across the nasal mucosa for delivery to the receptor. bOBP is required for transport because common odorants are traditionally hydrophobic and thus repelled by the hydrophilic layer. To accomplish its many tasks, it binds odorants in a hydrophobic β barrel motif. | Bovine odorant binding protein is a small homodimeric protein found in the nasal mucosa of cows; it has numerous possible functions, including odorant buffering, cellular transduction, insect repellant, and its most important function: odorant transport across the nasal mucosa for delivery to the receptor. bOBP is required for transport because common odorants are traditionally hydrophobic and thus repelled by the hydrophilic layer. To accomplish its many tasks, it binds odorants in a hydrophobic β barrel motif. | ||
| - | + | </StructureSection> | |
=='''References'''== | =='''References'''== | ||
<references /> | <references /> | ||
Current revision
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Pelosi P. Odorant-binding proteins. Crit Rev Biochem Mol Biol. 1994;29(3):199-228. PMID:8070277 doi:http://dx.doi.org/10.3109/10409239409086801
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Tegoni M, Pelosi P, Vincent F, Spinelli S, Campanacci V, Grolli S, Ramoni R, Cambillau C. Mammalian odorant binding proteins. Biochim Biophys Acta. 2000 Oct 18;1482(1-2):229-40. PMID:11058764
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Pevsner J, Hou V, Snowman AM, Snyder SH. Odorant-binding protein. Characterization of ligand binding. J Biol Chem. 1990 Apr 15;265(11):6118-25. PMID:2318850
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Tegoni M, Ramoni R, Bignetti E, Spinelli S, Cambillau C. Domain swapping creates a third putative combining site in bovine odorant binding protein dimer. Nat Struct Biol. 1996 Oct;3(10):863-7. PMID:8836103
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Bianchet MA, Bains G, Pelosi P, Pevsner J, Snyder SH, Monaco HL, Amzel LM. The three-dimensional structure of bovine odorant binding protein and its mechanism of odor recognition. Nat Struct Biol. 1996 Nov;3(11):934-9. PMID:8901871
- ↑ Vincent F, Ramoni R, Spinelli S, Grolli S, Tegoni M, Cambillau C. Crystal structures of bovine odorant-binding protein in complex with odorant molecules. Eur J Biochem. 2004 Oct;271(19):3832-42. PMID:15373829 doi:10.1111/j.1432-1033.2004.04315.x
- ↑ Borysik AJ, Briand L, Taylor AJ, Scott DJ. Rapid odorant release in mammalian odour binding proteins facilitates their temporal coupling to odorant signals. J Mol Biol. 2010 Dec 3;404(3):372-80. Epub 2010 Oct 7. PMID:20932975 doi:10.1016/j.jmb.2010.09.019
- ↑ Ramoni R, Vincent F, Grolli S, Conti V, Malosse C, Boyer FD, Nagnan-Le Meillour P, Spinelli S, Cambillau C, Tegoni M. The insect attractant 1-octen-3-ol is the natural ligand of bovine odorant-binding protein. J Biol Chem. 2001 Mar 9;276(10):7150-5. Epub 2000 Dec 12. PMID:11114310 doi:http://dx.doi.org/10.1074/jbc.M010368200
- ↑ Ramoni R, Spinelli S, Grolli S, Conti V, Merli E, Cambillau C, Tegoni M. Deswapping bovine odorant binding protein. Biochim Biophys Acta. 2008 Apr;1784(4):651-7. Epub 2008 Jan 29. PMID:18269920 doi:10.1016/j.bbapap.2008.01.010
- Articles 1 and 2 are review articles
Contributors
Special thanks to Professor David Nelson and Professor Michael Patrick for their research guidance and technical expertise in putting these pages together.