1ygp
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:1ygp.png|left|200px]] | ||
| - | + | ==PHOSPHORYLATED FORM OF YEAST GLYCOGEN PHOSPHORYLASE WITH PHOSPHATE BOUND IN THE ACTIVE SITE.== | |
| + | <StructureSection load='1ygp' size='340' side='right'caption='[[1ygp]], [[Resolution|resolution]] 2.80Å' scene=''> | ||
| + | == Structural highlights == | ||
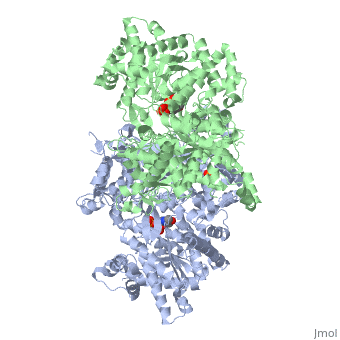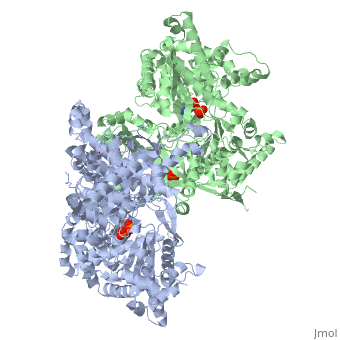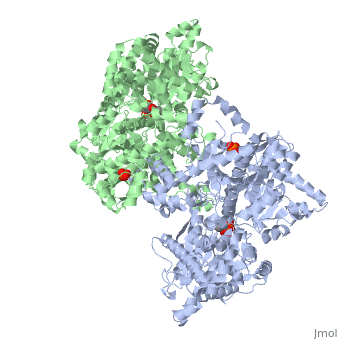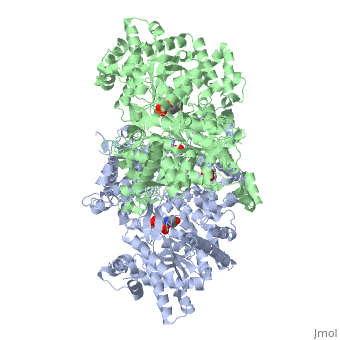
| + | <table><tr><td colspan='2'>[[1ygp]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. The December 2001 RCSB PDB [https://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Glycogen Phosphorylase'' by David S. Goodsell is [https://dx.doi.org/10.2210/rcsb_pdb/mom_2001_12 10.2210/rcsb_pdb/mom_2001_12]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1YGP OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1YGP FirstGlance]. <br> | ||
| + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.8Å</td></tr> | ||
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=PLP:PYRIDOXAL-5-PHOSPHATE'>PLP</scene>, <scene name='pdbligand=PO4:PHOSPHATE+ION'>PO4</scene></td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1ygp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1ygp OCA], [https://pdbe.org/1ygp PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1ygp RCSB], [https://www.ebi.ac.uk/pdbsum/1ygp PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1ygp ProSAT]</span></td></tr> | ||
| + | </table> | ||
| + | == Function == | ||
| + | [https://www.uniprot.org/uniprot/PHSG_YEAST PHSG_YEAST] Phosphorylase is an important allosteric enzyme in carbohydrate metabolism. Enzymes from different sources differ in their regulatory mechanisms and in their natural substrates. However, all known phosphorylases share catalytic and structural properties. | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/yg/1ygp_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1ygp ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
| + | A phosphorylation-initiated mechanism of local protein refolding activates yeast glycogen phosphorylase (GP). Refolding of the phosphorylated amino-terminus was shown to create a hydrophobic cluster that wedges into the subunit interface of the enzyme to trigger activation. The phosphorylated threonine is buried in the allosteric site. The mechanism implicates glucose 6-phosphate, the allosteric inhibitor, in facilitating dephosphorylation by dislodging the buried covalent phosphate through binding competition. Thus, protein phosphorylation-dephosphorylation may also be controlled through regulation of the accessibility of the phosphorylation site to kinases and phosphatases. In mammalian glycogen phosphorylase, phosphorylation occurs at a distinct locus. The corresponding allosteric site binds a ligand activator, adenosine monophosphate, which triggers activation by a mechanism analogous to that of phosphorylation in the yeast enzyme. | ||
| - | + | A protein phosphorylation switch at the conserved allosteric site in GP.,Lin K, Rath VL, Dai SC, Fletterick RJ, Hwang PK Science. 1996 Sep 13;273(5281):1539-42. PMID:8703213<ref>PMID:8703213</ref> | |
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| - | + | <div class="pdbe-citations 1ygp" style="background-color:#fffaf0;"></div> | |
| - | + | ||
==See Also== | ==See Also== | ||
*[[Glycogen Phosphorylase|Glycogen Phosphorylase]] | *[[Glycogen Phosphorylase|Glycogen Phosphorylase]] | ||
| - | + | *[[Glycogen phosphorylase 3D structures|Glycogen phosphorylase 3D structures]] | |
| - | == | + | == References == |
| - | < | + | <references/> |
| + | __TOC__ | ||
| + | </StructureSection> | ||
[[Category: Glycogen Phosphorylase]] | [[Category: Glycogen Phosphorylase]] | ||
| + | [[Category: Large Structures]] | ||
[[Category: RCSB PDB Molecule of the Month]] | [[Category: RCSB PDB Molecule of the Month]] | ||
[[Category: Saccharomyces cerevisiae]] | [[Category: Saccharomyces cerevisiae]] | ||
| - | [[Category: Dai | + | [[Category: Dai SC]] |
| - | [[Category: Fletterick | + | [[Category: Fletterick RJ]] |
| - | [[Category: Hwang | + | [[Category: Hwang PK]] |
| - | [[Category: Lin | + | [[Category: Lin K]] |
| - | [[Category: Rath | + | [[Category: Rath VL]] |
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
PHOSPHORYLATED FORM OF YEAST GLYCOGEN PHOSPHORYLASE WITH PHOSPHATE BOUND IN THE ACTIVE SITE.
| |||||||||||