Leishmania infantum Glyoxalase II
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
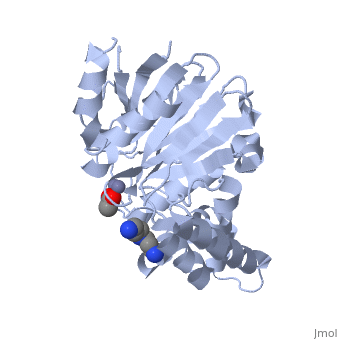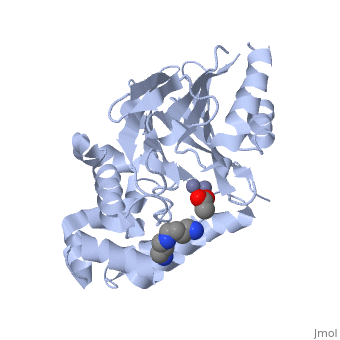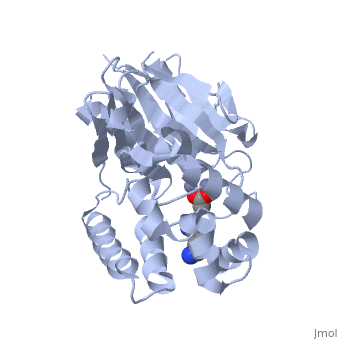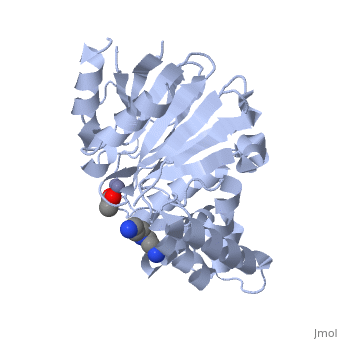
| - | <StructureSection load="2p18" size="400" color="" frame="true" spin="on" Scene= | + | <StructureSection load="2p18" size="400" color="" frame="true" spin="on" Scene= side="right" caption="Glyoxalase II complex with spermidine, acetate and Zn+2 ion, [[2p18]]" > |
[[Image:2p18.jpg|left|200px]] | [[Image:2p18.jpg|left|200px]] | ||
| Line 9: | Line 9: | ||
==Overview== | ==Overview== | ||
| - | The glyoxalase pathway catalyzes the formation of D-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. | + | The '''glyoxalase''' pathway catalyzes the formation of D-lactate from methylglyoxal, a toxic byproduct of glycolysis. In trypanosomatids, trypanothione replaces glutathione in this pathway, making it a potential drug target, since its selective inhibition might increase methylglyoxal concentration in the parasites. |
Evolutionary analysis shows that trypanosomatid ''L. infantum'' glyoxalase II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins. This enzyme shows absolute specificity towards trypanothione, making it an exceptional model to understand the molecular basis of trypanothione binding and specificity. | Evolutionary analysis shows that trypanosomatid ''L. infantum'' glyoxalase II diverged early from eukaryotic enzymes, being unrelated to prokaryotic proteins. This enzyme shows absolute specificity towards trypanothione, making it an exceptional model to understand the molecular basis of trypanothione binding and specificity. | ||
| Line 32: | Line 32: | ||
Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P18 OCA]. | Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2P18 OCA]. | ||
| - | + | ||
| + | ==3D structure of glyoxalase== | ||
| + | |||
| + | [[Glyoxalase]] | ||
| + | |||
| + | |||
==Reference== | ==Reference== | ||
Catalysis and structural properties of Leishmania infantum glyoxalase II: trypanothione specificity and phylogeny., Sousa Silva M, Barata L, Ferreira AE, Romao S, Tomas AM, Ponces Freire A, Cordeiro C, Biochemistry. 2008 Jan 8;47(1):195-204. Epub 2007 Dec 6. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/18052346 18052346] | Catalysis and structural properties of Leishmania infantum glyoxalase II: trypanothione specificity and phylogeny., Sousa Silva M, Barata L, Ferreira AE, Romao S, Tomas AM, Ponces Freire A, Cordeiro C, Biochemistry. 2008 Jan 8;47(1):195-204. Epub 2007 Dec 6. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/18052346 18052346] | ||
Current revision
| |||||||||||