G13secL03Tpc3
From Proteopedia
| (41 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Outer Surface Protein B of ''Borrelia burgdorferi'' == | == Outer Surface Protein B of ''Borrelia burgdorferi'' == | ||
| - | |||
| - | Anything in this section will appear adjacent to the 3D structure and will be scrollable. | ||
| - | |||
| - | </StructureSection> | ||
| - | |||
| - | <Structure load='1RJL' size='500' frame='true' align='right' caption='Insert caption here' scene= G13secL03Tpc3/Ospb_c_terminus/2> | ||
| - | |||
==Introduction== | ==Introduction== | ||
| - | [http://en.wikipedia.org/wiki/Borrelia_burgdorferi ''Borrelia burgdorferi'']are motile spirochetes that | + | |
| + | [http://en.wikipedia.org/wiki/Borrelia_burgdorferi ''Borrelia burgdorferi'']are motile spirochetes that reside in the gut of hard- bodied deer tick of the family ''Ixodidae''. They are transmitted in humans by the bite of this tick. [http://en.wikipedia.org/wiki/Lyme_disease Lyme disease], caused by ''Borrelia burgdorferi'', is involved in many serious disorders of the skin, nervous and cardiac system. The bacterium consists of outer surface proteins that play an important role in helping the bacteria colonize and survive in the tick. Of these, a major lipoprotein is Osp B. This outer surface protein has a property of becoming lysed without a direct complement with the antibody. Specifically, the C terminus on the Osp B contains a Lysine residue that recognizes the destructive IgG antibody, H6831. <ref name=Article1> PMID:7513309 </ref> The structure and stability of Osp B is affected by this antibody causing the bacteria to lyse. | ||
==Structure== | ==Structure== | ||
| + | <Structure load='1RJL' size='350' frame='true' align='right' scene='G13secL03Tpc3/Final_ospb/2'/> | ||
| + | <Structure load='1RJL' size='350' frame='true' align='right' scene='G13secL03Tpc3/Final_bsheet/2'/> | ||
| - | Osp B extends from Serine- 157 to Lysine- 296. It consists of twelve anti parallel beta sheets and one alpha helix. Sheets 1- 4 form a freestanding sheet, where sheets 5-12 form a barrel like domain. The Fab binding domain is in the center of the central beta sheet, residues 152-296. | ||
| - | ==Interactions== | + | Osp B extends from Serine- 157 to Lysine- 296. It consists of twelve anti parallel beta sheets and one alpha helix. Sheets 1- 4 form a freestanding sheet, where sheets 5-12 form a barrel like domain. The Fab binding domain is in the center of the central beta sheet, residues 152-296.<ref name=Article2> PMID:15713683 </ref> |
| - | The structure of Osp B shows that there are three exposed loops | + | |
| + | ===Interactions=== | ||
| + | The structure of Osp B shows that there are three exposed loops that are affected by the antibody, H6831. The majority of interaction occurs between the antibody’s heavy chain and <scene name='G13secL03Tpc3/Loop_2_final/1'>Loop 2</scene> on the lipoprotein, residues 250-254. The loop forms electrostatic interaction and hydrogen bond interactions with the antibody's heavy chain. Lysine 253, which resides on Loop 2, plays an important role in the recognition of the antibody, thus the antibody having the most interaction with loop 2. The Lysine interacts with the antibody by forming hydrogen bonds with the glutamine and histidine on the heavy chain of H6831. <scene name='G13secL03Tpc3/Loop_1/1'>Loop 1</scene>, residues 231-233, also interacts with the Fab heavy chain by forming two hydrogen bonds. <scene name='G13secL03Tpc3/Loop_3_final/2'>Loop 3</scene>, residues 272-276, interacts with the Fab light chain of the antibody with two hydrogen bonds. <ref name=Article2> PMID:15864264 </ref> Loop 3 contains Threonine 276, an amino acid that, along with Lysine 253, contributes most to the contact and binding of H6831 to OspB. | ||
==Structural changes== | ==Structural changes== | ||
| - | + | A more significant <scene name='G13secL03Tpc3/Final_bsheet/3'>structural change</scene> that occurs during the bactericidal Fab complex is the removal of OspB's first four beta sheets. Surrounding sheets shift and overlap to replace the missing sheets. The removal of these sheets can affect the outer surface protein and make the bacteria more susceptible to lysis. | |
=== Destruction=== | === Destruction=== | ||
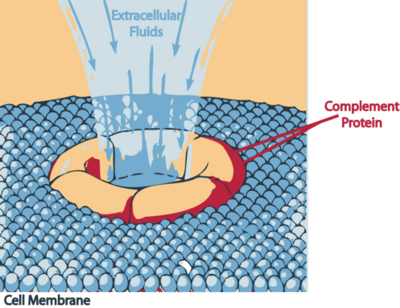
| - | The bacteria lyses indirectly | + | The bacteria lyses indirectly. The proteolytic activity of the antibody on the bacteria is presumably similar to a [http://en.wikipedia.org/wiki/Complement_membrane_attack_complex membrane attack complex] (MAC). The complement system activates the classical pathway, activating effector proteins which insert themselves in the outer membrane of the bacteria and disrupting it and causing death to the cell. <ref name=Article3> PMID:15864264 </ref> |
| + | [[Image:Mac attack.PNG|thumb|400px|Mechanism of destruction by MAC on the bacteria, ''Borrelia burgdorferi'']] | ||
| + | |||
| + | ==Application== | ||
| + | Under pressure in the presence of antibodies, OspB is capable of existing in various forms. Mutations occur in the residue of Lysine 253, the epitope of H6831, and may prevent ''Borrelia'' from being recognized and lysed by bactericidal Fabs such as H6381. <ref name=Article4> PMID: 15128807 </ref> The study of this structure of Osp B is important to combat future mechanisms of curing Lyme disease. Vaccines created to specifically fight OspB have not yet been successful, due to the increased variability of the epitope. Vaccine are not yet able to counteract the varied strains of ''Borrelia burgdorferi.'' However, the ability of H6381 and CB2 to make the bacteria subject to increased proteolytic activity is a possibility for future research, as it is still currently misunderstood. | ||
| + | |||
| + | |||
| + | |||
| + | References: {{reflist}} | ||
Current revision
Contents |
Outer Surface Protein B of Borrelia burgdorferi
Introduction
Borrelia burgdorferiare motile spirochetes that reside in the gut of hard- bodied deer tick of the family Ixodidae. They are transmitted in humans by the bite of this tick. Lyme disease, caused by Borrelia burgdorferi, is involved in many serious disorders of the skin, nervous and cardiac system. The bacterium consists of outer surface proteins that play an important role in helping the bacteria colonize and survive in the tick. Of these, a major lipoprotein is Osp B. This outer surface protein has a property of becoming lysed without a direct complement with the antibody. Specifically, the C terminus on the Osp B contains a Lysine residue that recognizes the destructive IgG antibody, H6831. [1] The structure and stability of Osp B is affected by this antibody causing the bacteria to lyse.
Structure
|
|
Osp B extends from Serine- 157 to Lysine- 296. It consists of twelve anti parallel beta sheets and one alpha helix. Sheets 1- 4 form a freestanding sheet, where sheets 5-12 form a barrel like domain. The Fab binding domain is in the center of the central beta sheet, residues 152-296.[2]
Interactions
The structure of Osp B shows that there are three exposed loops that are affected by the antibody, H6831. The majority of interaction occurs between the antibody’s heavy chain and on the lipoprotein, residues 250-254. The loop forms electrostatic interaction and hydrogen bond interactions with the antibody's heavy chain. Lysine 253, which resides on Loop 2, plays an important role in the recognition of the antibody, thus the antibody having the most interaction with loop 2. The Lysine interacts with the antibody by forming hydrogen bonds with the glutamine and histidine on the heavy chain of H6831. , residues 231-233, also interacts with the Fab heavy chain by forming two hydrogen bonds. , residues 272-276, interacts with the Fab light chain of the antibody with two hydrogen bonds. [2] Loop 3 contains Threonine 276, an amino acid that, along with Lysine 253, contributes most to the contact and binding of H6831 to OspB.
Structural changes
A more significant that occurs during the bactericidal Fab complex is the removal of OspB's first four beta sheets. Surrounding sheets shift and overlap to replace the missing sheets. The removal of these sheets can affect the outer surface protein and make the bacteria more susceptible to lysis.
Destruction
The bacteria lyses indirectly. The proteolytic activity of the antibody on the bacteria is presumably similar to a membrane attack complex (MAC). The complement system activates the classical pathway, activating effector proteins which insert themselves in the outer membrane of the bacteria and disrupting it and causing death to the cell. [3]
Application
Under pressure in the presence of antibodies, OspB is capable of existing in various forms. Mutations occur in the residue of Lysine 253, the epitope of H6831, and may prevent Borrelia from being recognized and lysed by bactericidal Fabs such as H6381. [4] The study of this structure of Osp B is important to combat future mechanisms of curing Lyme disease. Vaccines created to specifically fight OspB have not yet been successful, due to the increased variability of the epitope. Vaccine are not yet able to counteract the varied strains of Borrelia burgdorferi. However, the ability of H6381 and CB2 to make the bacteria subject to increased proteolytic activity is a possibility for future research, as it is still currently misunderstood.
- ↑ Sadziene A, Jonsson M, Bergstrom S, Bright RK, Kennedy RC, Barbour AG. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect Immun. 1994 May;62(5):2037-45. PMID:7513309
- ↑ 2.0 2.1 Becker M, Bunikis J, Lade BD, Dunn JJ, Barbour AG, Lawson CL. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J Biol Chem. 2005 Apr 29;280(17):17363-70. Epub 2005 Feb 15. PMID:15713683 doi:10.1074/jbc.M412842200
- ↑ Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005 May;3(5):411-20. PMID:15864264 doi:10.1038/nrmicro1149
- ↑ Alitalo A, Meri T, Chen T, Lankinen H, Cheng ZZ, Jokiranta TS, Seppala IJ, Lahdenne P, Hefty PS, Akins DR, Meri S. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J Immunol. 2004 May 15;172(10):6195-201. PMID:15128807