We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Galectin
From Proteopedia
(Difference between revisions)
| (23 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
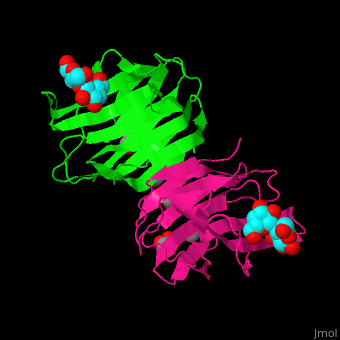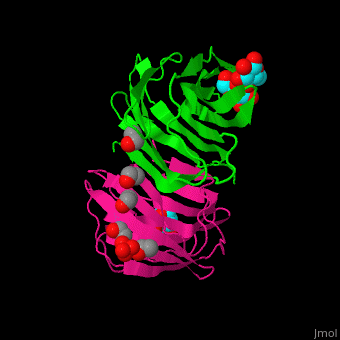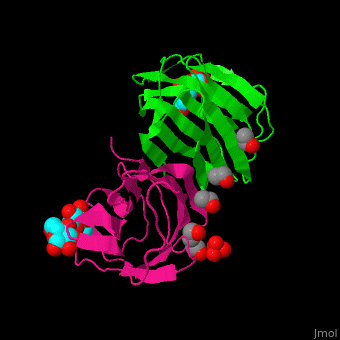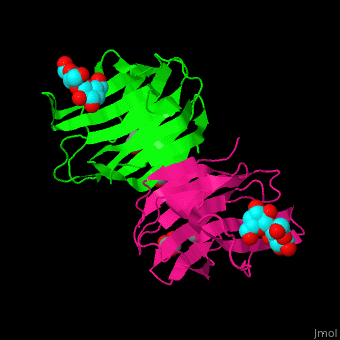
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Structure of human galectin-1 complex with lactose, mercaptoethanol and sulfate (PDB entry [[1w6o]])' scene='51/516488/Cv/1'> |
| + | __TOC__ | ||
| + | == Function == | ||
| - | '''Galectin''' (GAL) are lectins which bind | + | '''Galectin''' or '''galactoside-binding soluble lectin''' (GAL) are lectins which bind β-galactosidase (BGAL). GAL contains a carbohydrate recognition domain (CRD) residues 113-250<ref>PMID:8063692</ref>. <br /> |
| + | * GAL-1 is thought to play a role as autocrine negative growth factor that regulates cell proliferation.<br /> | ||
| + | * GAL-2 is expressed primarily in gastrointestinal tract.<br /> | ||
| + | * GAL-3 has broad biological functionality.<br /> | ||
| + | * GAL-4 has 2 CRDs.<br /> | ||
| + | * GAL-5 is found in rat is a β-galactoside binding lectin<ref>PMID:19903899</ref>.<br /> | ||
| + | * GAL-7 is associated with epithelial cells.<br /> | ||
| + | * GAL-8 has a role in cellular defense against bacterial infection and vacuolar damage.<br /> | ||
| + | * GAL-9 affects eosinophil survival. <br /> | ||
| + | * GAL-10 or Charcot-Leyden crystal protein acts on biological membranes to regulate the multifunctional lysophospholipids.<br /> | ||
| + | * GAL-13 is a placental protein which does not bind β-galactosides.<br /> | ||
| - | == | + | == Relevance == |
| - | + | GAL are known to inhibit chronic inflammation<ref>PMID:20146714</ref>. GAL-3 has a role in experimental autoimmune encephalomyelitis and melanoma methastasis<ref>PMID:22418727</ref>. | |
| - | + | == Structural highlights == | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | = | + | The CRD is a β-sandwich long enough to bind a tetrasaccharide. A <scene name='51/516488/Cv/4'>lactose ligand is bound at the concave face of the β-sheet</scene><ref>PMID:15476813</ref>. Water molecules shown as red spheres. |
| - | + | ==3D structures of galectin== | |
| - | [[ | + | [[Galectin 3D structures]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </StructureSection> | |
| - | + | == References == | |
| - | + | <references/> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | [[Category:Topic Page]] | |
| - | + | ||
| - | [[ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
| |||||||||||
References
- ↑ Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994 Aug 19;269(33):20807-10. PMID:8063692
- ↑ Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010 Jan 21;115(3):696-705. doi: 10.1182/blood-2009-07-231449. Epub 2009, Nov 10. PMID:19903899 doi:http://dx.doi.org/10.1182/blood-2009-07-231449
- ↑ Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010 Jan;1183:158-82. doi: 10.1111/j.1749-6632.2009.05131.x. PMID:20146714 doi:http://dx.doi.org/10.1111/j.1749-6632.2009.05131.x
- ↑ Radosavljevic G, Volarevic V, Jovanovic I, Milovanovic M, Pejnovic N, Arsenijevic N, Hsu DK, Lukic ML. The roles of Galectin-3 in autoimmunity and tumor progression. Immunol Res. 2012 Apr;52(1-2):100-10. doi: 10.1007/s12026-012-8286-6. PMID:22418727 doi:http://dx.doi.org/10.1007/s12026-012-8286-6
- ↑ Lopez-Lucendo MF, Solis D, Andre S, Hirabayashi J, Kasai K, Kaltner H, Gabius HJ, Romero A. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004 Oct 29;343(4):957-70. PMID:15476813 doi:http://dx.doi.org/10.1016/j.jmb.2004.08.078