We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
MtrF
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='3pmq' size='400' side='right' scene='' caption=''MtrF General Structure containing 10 heme groups, pdb:[[3pmq]]''> |
| + | |||
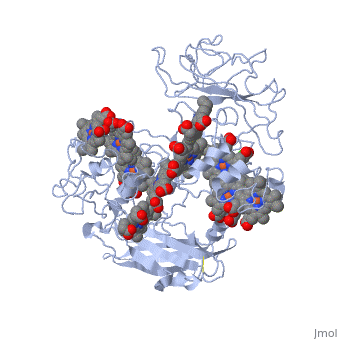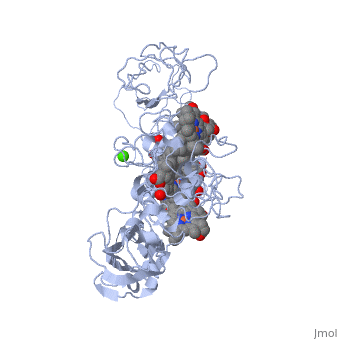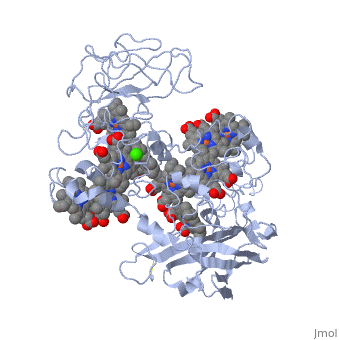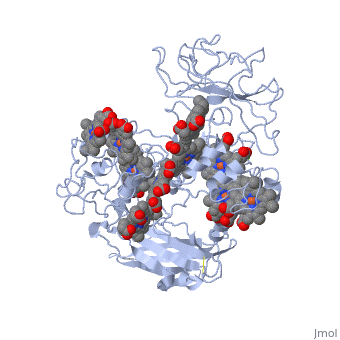
[[MtrF]] is a cell surface cytochrome on the Gram-negative bacteria known as ''Shewanella oneidensis''.<ref name="mtrf"> PMID:11418600</ref> MtrF is involved with shuttling electrons across its (''S. oneidensis'') outer surface. MtrF has several homologues, MtrC and the protein OmcA. These three different proteins are thought to be replaceable with one another in deletion mutation experiments.<ref name= "mtrf" /> | [[MtrF]] is a cell surface cytochrome on the Gram-negative bacteria known as ''Shewanella oneidensis''.<ref name="mtrf"> PMID:11418600</ref> MtrF is involved with shuttling electrons across its (''S. oneidensis'') outer surface. MtrF has several homologues, MtrC and the protein OmcA. These three different proteins are thought to be replaceable with one another in deletion mutation experiments.<ref name= "mtrf" /> | ||
__TOC__ | __TOC__ | ||
| Line 15: | Line 16: | ||
A prime example of MtrF regulation is the study of "Regulation of MtrF Expression in ''Neisseria gonorrhoeae'' and Its Role in High-Level Antimicrobial Resistance".<ref> PMID:15901695 </ref> In this study the expression of MtrF was repressed by MtrR (the major repressor in the mtrCDE expression). Another repressor known as MpeR can also regulate the expression of MtrF. Repression of MtrF by MtrR and MpeR was used, demonstrating that the repressive effects mediated by these regulators are independent processes. MtrF was also disabled and the significant reduction in the induction of hydrophobic agent resistance and it was found that the expression of MtrF is enhanced when ''Gonococci'' are grown under inducing conditions. | A prime example of MtrF regulation is the study of "Regulation of MtrF Expression in ''Neisseria gonorrhoeae'' and Its Role in High-Level Antimicrobial Resistance".<ref> PMID:15901695 </ref> In this study the expression of MtrF was repressed by MtrR (the major repressor in the mtrCDE expression). Another repressor known as MpeR can also regulate the expression of MtrF. Repression of MtrF by MtrR and MpeR was used, demonstrating that the repressive effects mediated by these regulators are independent processes. MtrF was also disabled and the significant reduction in the induction of hydrophobic agent resistance and it was found that the expression of MtrF is enhanced when ''Gonococci'' are grown under inducing conditions. | ||
| + | </StructureSection> | ||
==3D Structures of Cytochrome C== | ==3D Structures of Cytochrome C== | ||
[[Cytochrome c]] | [[Cytochrome c]] | ||
Current revision
| |||||||||||
3D Structures of Cytochrome C
References
- ↑ 1.0 1.1 1.2 1.3 Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001 Aug 24;276(34):32274-81. Epub 2001 Jun 19. PMID:11418600 doi:10.1074/jbc.M103285200
- ↑ Veal WL, Shafer WM. Identification of a cell envelope protein (MtrF) involved in hydrophobic antimicrobial resistance in Neisseria gonorrhoeae. J Antimicrob Chemother. 2003 Jan;51(1):27-37. PMID:12493784
- ↑ Folster JP, Shafer WM. Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance. J Bacteriol. 2005 Jun;187(11):3713-20. PMID:15901695 doi:10.1128/JB.187.11.3713-3720.2005
Proteopedia Page Contributors and Editors (what is this?)
David Canner, Michal Harel, Holly Huntley, Alexander Berchansky