This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
MDM2
From Proteopedia
(Difference between revisions)
| (13 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='2axi' size='350' side='right' scene='' caption='Human MDM2 (grey) complex with β-hairpin peptide (green), sulfate and morpholino-propane sulfonic acid [[1ycq]]'> | |
| + | __TOC__ | ||
| + | ==Function== | ||
| - | + | The '''MDM2''' or '''E3 ubiquitin-protein ligase MDM2''' oncoprotein is a cellular inhibitor of the [[P53|p53 tumor suppressor]] in that it can bind the transactivation domain of p53 and downregulate its ability to activate transcription. In certain [[Cancer|cancers]], MDM2 amplification is a common event and contributes to the inactivation of p53. The crystal structure of the 109-residue amino-terminal domain of MDM2 bound to 109-residue transactivation domain peptide of p53 revealed that MDM2 has a <scene name='Matt_Routh_sandbox/Hydrophobic_cavity/1'>deep hydrophobic cleft</scene> on which the p53 peptide binds as an amphipathic alpha helix. The interface relies on the steric complementarity between the MDM2 cleft and the hydrophobic face of the p53 alpha helix and, in particular, on a triad of p53 amino acids-Phe19, Trp23, and Leu26-which insert <scene name='Matt_Routh_sandbox/Connection_to_p53/5'>deep inside</scene> the MDM2 cleft. These same p53 residues are also involved in transactivation, supporting the hypothesis that MDM2 inactivates p53 by concealing its transactivation domain. The structure also suggests that the amphipathic alpha helix may be a common structural motif in the binding of a diverse family of transactivation factors to the TATA-binding protein-associated factors.<ref name="Kussie">PMID:8875929</ref> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | The '''MDM2''' oncoprotein is a cellular inhibitor of the [[P53|p53 tumor suppressor]] in that it can bind the transactivation domain of p53 and downregulate its ability to activate transcription. In certain [[Cancer|cancers]], MDM2 amplification is a common event and contributes to the inactivation of p53. The crystal structure of the 109-residue amino-terminal domain of MDM2 bound to 109-residue transactivation domain peptide of p53 revealed that MDM2 has a <scene name='Matt_Routh_sandbox/Hydrophobic_cavity/1'>deep hydrophobic cleft</scene> on which the p53 peptide binds as an amphipathic alpha helix. The interface relies on the steric complementarity between the MDM2 cleft and the hydrophobic face of the p53 alpha helix and, in particular, on a triad of p53 amino acids-Phe19, Trp23, and Leu26-which insert <scene name='Matt_Routh_sandbox/Connection_to_p53/5'>deep inside</scene> the MDM2 cleft. These same p53 residues are also involved in transactivation, supporting the hypothesis that MDM2 inactivates p53 by concealing its transactivation domain. The structure also suggests that the amphipathic alpha helix may be a common structural motif in the binding of a diverse family of transactivation factors to the TATA-binding protein-associated factors.<ref name="Kussie">PMID:8875929</ref> | + | |
==Role in Cancer== | ==Role in Cancer== | ||
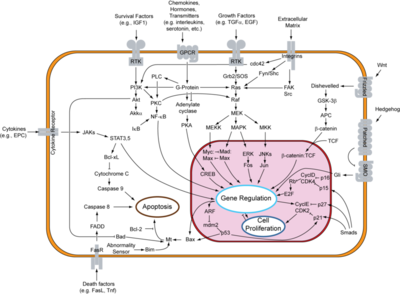
[[Image:800px-Signal transduction v1.png|Apoptosis signal pathway|400 px|thumb]] | [[Image:800px-Signal transduction v1.png|Apoptosis signal pathway|400 px|thumb]] | ||
MDM2 is an active inhibitor of the p53 anti-tumorgenesis protein. When MDM2 is atached p53 is unable to release transcription factors that code for apoptosis or halting of the cell cycle. The full cell signaling pathway is very complex, but MDM2 overproduction has been shown to be connected to cancer activity.<ref name="Pollock">PMID:18521197</ref> Genetic amplification of MDM2 has been shown in breast, glioma, pancreaticadenocarcinoma, leucemias, Hodgkin's and nonhodgkin's lymphomas and other various cancers.<ref name="Pollock"/> This broad observation outlines the importance of determining MDM2's role in tumer-genesis and how the process can be treated. | MDM2 is an active inhibitor of the p53 anti-tumorgenesis protein. When MDM2 is atached p53 is unable to release transcription factors that code for apoptosis or halting of the cell cycle. The full cell signaling pathway is very complex, but MDM2 overproduction has been shown to be connected to cancer activity.<ref name="Pollock">PMID:18521197</ref> Genetic amplification of MDM2 has been shown in breast, glioma, pancreaticadenocarcinoma, leucemias, Hodgkin's and nonhodgkin's lymphomas and other various cancers.<ref name="Pollock"/> This broad observation outlines the importance of determining MDM2's role in tumer-genesis and how the process can be treated. | ||
| + | |||
| + | See also [[Proteins involved in cancer]]. | ||
==Experimental Research== | ==Experimental Research== | ||
| Line 39: | Line 18: | ||
==3D structures of MDM2== | ==3D structures of MDM2== | ||
| + | [[MDM2 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Resources== | ==Additional Resources== | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Cancer
Reference
- ↑ Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996 Nov 8;274(5289):948-53. PMID:8875929
- ↑ 2.0 2.1 2.2 Pollock RE, Lang A, El-Naggar AK, Radinsky R, Hung MC. Enhanced MDM2 Oncoprotein Expression in Soft Tissue Sarcoma: Several Possible Regulatory Mechanisms. Sarcoma. 1997;1(1):23-9. PMID:18521197 doi:10.1080/13577149778443
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Matt Routh, David Canner, Joel L. Sussman, Yousuf Bahrami, Alexander Berchansky