We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Fragment-Based Drug Discovery
From Proteopedia
(Difference between revisions)
m (Sandbox reserved 394 moved to Fragment-Based Drug Discovery: requested by Authors) |
|||
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
= Drug Design: Fragment-Based Drug Discovery = | = Drug Design: Fragment-Based Drug Discovery = | ||
| - | <StructureSection load='' size='500' side='right' caption='Bcl-xl in complex with ABT-737 (PDB entry [[2yxj]])' scene='Sandbox_reserved_394/Bcl-xl_abt-737_complex/ | + | <StructureSection load='' size='500' side='right' caption='Bcl-xl in complex with ABT-737 (PDB entry [[2yxj]])' scene='Sandbox_reserved_394/Bcl-xl_abt-737_complex/6'> |
Traditionally, new drugs are developed by either making small changes to existing drugs or by individually testing thousands of compounds. Both of these methods require many hours of laborious chemical synthesis. However, new techniques are being applied in the drug industry which show promise in decreasing the cost and time required to discover and develop new drugs. | Traditionally, new drugs are developed by either making small changes to existing drugs or by individually testing thousands of compounds. Both of these methods require many hours of laborious chemical synthesis. However, new techniques are being applied in the drug industry which show promise in decreasing the cost and time required to discover and develop new drugs. | ||
| Line 13: | Line 13: | ||
| - | The development of <scene name='Sandbox_reserved_394/Abt-737/ | + | The development of <scene name='Sandbox_reserved_394/Abt-737/6'>ABT-737</scene> using SAR by NMR is a classic example of FBDD. (Throughout this discussion ABT-737 will be used to illustrate the FBDD process.) This compound has been shown to effectively inhibit the over-expression of <scene name='Sandbox_reserved_394/Bcl-xl/1'>Bcl-xl</scene> which is a protein that is commonly observed to be over-expressed in many types of cancers.<ref name="Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579">Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579</ref> It acts an inhibitor of apoptosis and may also contribute to chemotherapy resistance. Bcl-xl inhibition by ABT-737 therefore, allows apoptosis to occur and helps to prevent chemo-resistance. |
{| class="wikitable collapsible" | {| class="wikitable collapsible" | ||
! scope="col" width="5000px" | SAR by NMR | ! scope="col" width="5000px" | SAR by NMR | ||
|- | |- | ||
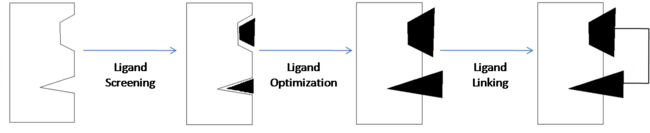
| - | | scope="col" width="5000px" | Structure-activity relationship (SAR) by NMR is one tool that can be used to design and develop new drugs. This is the process | + | | scope="col" width="5000px" | Structure-activity relationship (SAR) by NMR is one tool that can be used to design and develop new drugs. This is the process in which NMR is used to identify the components responsible for binding to the protein. NMR is also used to analyze the relationships between these components to determine where the protein binding sites are located and how the ligand interacts with those sites.<ref name="Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.">Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.</ref> |
|} | |} | ||
| Line 26: | Line 26: | ||
===== ABT-737: ligand screening ===== | ===== ABT-737: ligand screening ===== | ||
| - | <scene name='Sandbox_reserved_394/Compound_1/ | + | <scene name='Sandbox_reserved_394/Compound_1/12'>Two fragments</scene> were found to have moderate affinity for Bcl-xl. <scene name='Sandbox_reserved_394/Compound_1/9'>Compound 1</scene> is a fluorobiphenylcarboxylic acid. It occupies <scene name='Sandbox_reserved_394/Binding_site_1/4'>binding site 1</scene> of Bcl-xl which consists of Phe 101, Tyr 105, Ala 108, Phe 109, Leu 136, Gly 142, Arg 143, and Ala 146. The fluorobiphenyl system of compound 1 is very hydrophobic and therefore, these residues form a <scene name='Sandbox_reserved_394/Compound_1/4'>"hydrophobic pocket"</scene> around the system. There is also one hydrophilic interaction involved in this complex. The <scene name='Sandbox_reserved_394/Compound_1/5'>carboxylic acid portion of compound 1 binds near Gly 142</scene> of Bcl-xl. This is not a strong interaction but is significant because it can be modified to form a much stronger bond. |
<scene name='Sandbox_reserved_394/Compound_1/3'>Compound 2</scene> is a napthalene-based alcohol which occupies <scene name='Sandbox_reserved_394/Binding_site_2/4'>binding site 2</scene>. This particular fragment also is involved with hydrophobic interactions with Bcl-xl, although they are not as strong as in the case of compound 1. This binding site includes Ala 97, Glu 100, Phe 101, Val 145, and Tyr 199. | <scene name='Sandbox_reserved_394/Compound_1/3'>Compound 2</scene> is a napthalene-based alcohol which occupies <scene name='Sandbox_reserved_394/Binding_site_2/4'>binding site 2</scene>. This particular fragment also is involved with hydrophobic interactions with Bcl-xl, although they are not as strong as in the case of compound 1. This binding site includes Ala 97, Glu 100, Phe 101, Val 145, and Tyr 199. | ||
| Line 43: | Line 43: | ||
Compounds 1 & 2 exhibited very poor binding affinity for Bcl-xl. The optimization of these two compounds resulted in <scene name='Sandbox_reserved_394/Compound_2/1'>Compound 3</scene>. In order to improve the binding affinity, the carboxylic acid of compound 1 was substituted with an acyl sulfonamide to capitalize on the hydrophilic interaction with the protein. This <scene name='Sandbox_reserved_394/Compound_2/3'>acylsulfonamide portion forms a hydrogen bond with Gly 142</scene> thereby increasing the affinity for Bcl-xl. The substitution of the sulfonamide actually allows the acidic proton to get closer to Gly 142 than it could in the carboxylic acid, which is why it is able to bind stronger to the amino acid. | Compounds 1 & 2 exhibited very poor binding affinity for Bcl-xl. The optimization of these two compounds resulted in <scene name='Sandbox_reserved_394/Compound_2/1'>Compound 3</scene>. In order to improve the binding affinity, the carboxylic acid of compound 1 was substituted with an acyl sulfonamide to capitalize on the hydrophilic interaction with the protein. This <scene name='Sandbox_reserved_394/Compound_2/3'>acylsulfonamide portion forms a hydrogen bond with Gly 142</scene> thereby increasing the affinity for Bcl-xl. The substitution of the sulfonamide actually allows the acidic proton to get closer to Gly 142 than it could in the carboxylic acid, which is why it is able to bind stronger to the amino acid. | ||
| - | Compound 2 was important in identifying the hydrophobicity of binding site 2 but was | + | Compound 2 was important in identifying the hydrophobicity of binding site 2 but affinity was increased by substituting a <scene name='Sandbox_reserved_394/Nitro_thio_phenyl_sub/1'>3-nitro-4-(2-phenylthioethyl)aminophenyl group</scene>. This substitution more efficiently binds to site 2 through <scene name='Sandbox_reserved_394/Pi_stacking/3'>pi-pi interactions with Phe 101 and Tyr 199</scene>. This idea of using a known ligand to develop another ligand, and eventually drugs, is known as ligand-based drug design. |
{| class="wikitable collapsible" | {| class="wikitable collapsible" | ||
| Line 51: | Line 51: | ||
|} | |} | ||
| - | One challenge in drug delivery is bioavailability. The bioavailibility may be decreased due to non-specific protein binding. Therefore, compound 3 required further optimization because | + | One challenge in drug delivery is bioavailability. The bioavailibility may be decreased due to non-specific protein binding. Therefore, compound 3 required further optimization because Bcl-xl affinity is greatly reduced in the presence of human serum albumin (HSA). In order to decrease HSA affinity, and therefore increase Bcl-xl affinity, SAR by NMR was used to modify compound 3 by eliminating key binding groups of the compound to HSA without affecting Bcl-xl affinity. |
{| class="wikitable collapsible" | {| class="wikitable collapsible" | ||
! scope="col" width="5000px" | Modifying compound 3 to reduce HSA affinity | ! scope="col" width="5000px" | Modifying compound 3 to reduce HSA affinity | ||
|- | |- | ||
| - | | scope="col" width="5000px" | Compound 3 has high affinity for Bcl-xl but has an even higher affinity for HSA. For this reason, when HSA is present, compound 3 and similar ligands are more likely to bind to HSA thereby decreasing the amount that can bind with Bcl-xl. In order to decrease the affinity for HSA while maintaining affinity for Bcl-xl, SAR by NMR was used to compare compound 3 with a <scene name='Sandbox_reserved_394/Compound_3/1'>compound 4</scene> (thioethylamino-2,4-dimethylphenyl analogue), which also has high affinity for HSA. It was found that <scene name='Sandbox_reserved_394/Compound_3/ | + | | scope="col" width="5000px" | Compound 3 has high affinity for Bcl-xl but has an even higher affinity for HSA. For this reason, when HSA is present, compound 3 and similar ligands are more likely to bind to HSA thereby decreasing the amount that can bind with Bcl-xl. In order to decrease the affinity for HSA while maintaining affinity for Bcl-xl, SAR by NMR was used to compare compound 3 with a <scene name='Sandbox_reserved_394/Compound_3/1'>compound 4</scene> (thioethylamino-2,4-dimethylphenyl analogue), which also has high affinity for HSA. It was found that <scene name='Sandbox_reserved_394/Compound_3/3'>two hydrophobic portions</scene> of compound 4 had very strong hydrophobic interactions with HSA. Therefore, these portions in compound 3 were modified with polar substituents to decrease HSA affinity. To decrease hydrophobicity, the fluorobiphenyl system was substituted with a <scene name='Sandbox_reserved_394/Piperazine_ring/2'>piperazine ring</scene> and a <scene name='Sandbox_reserved_394/Abt-737/4'>2-dimethylaminoethyl group</scene> was added to the thioethylamino linkage group. |
|} | |} | ||
Current revision
Drug Design: Fragment-Based Drug Discovery
| |||||||||||
References
- ↑ 1.0 1.1 Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.
- ↑ Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579
- ↑ Pandit D. LIGAND-BASED DRUG DESIGN: I. CONFORMATIONAL STUDIES OF GBR 12909 ANALOGS AS COCAINE ANTAGONISTS; II. 3D-QSAR STUDIES OF SALVINORIN A ANALOGS AS εΑΡΡΑ OPIOID AGONISTS. http://archives.njit.edu/vol01/etd/2000s/2007/njit-etd2007-051/njit-etd2007-051.pdf