We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Fragment-Based Drug Discovery
From Proteopedia
(Difference between revisions)
m (Sandbox reserved 394 moved to Fragment-Based Drug Discovery: requested by Authors) |
|||
| (2 intermediate revisions not shown.) | |||
| Line 13: | Line 13: | ||
| - | The development of <scene name='Sandbox_reserved_394/Abt-737/ | + | The development of <scene name='Sandbox_reserved_394/Abt-737/6'>ABT-737</scene> using SAR by NMR is a classic example of FBDD. (Throughout this discussion ABT-737 will be used to illustrate the FBDD process.) This compound has been shown to effectively inhibit the over-expression of <scene name='Sandbox_reserved_394/Bcl-xl/1'>Bcl-xl</scene> which is a protein that is commonly observed to be over-expressed in many types of cancers.<ref name="Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579">Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579</ref> It acts an inhibitor of apoptosis and may also contribute to chemotherapy resistance. Bcl-xl inhibition by ABT-737 therefore, allows apoptosis to occur and helps to prevent chemo-resistance. |
{| class="wikitable collapsible" | {| class="wikitable collapsible" | ||
! scope="col" width="5000px" | SAR by NMR | ! scope="col" width="5000px" | SAR by NMR | ||
| Line 26: | Line 26: | ||
===== ABT-737: ligand screening ===== | ===== ABT-737: ligand screening ===== | ||
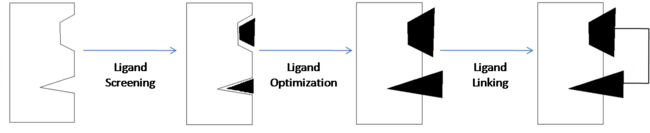
| - | <scene name='Sandbox_reserved_394/Compound_1/12'>Two fragments</scene> were found to have moderate affinity for Bcl-xl. <scene name='Sandbox_reserved_394/Compound_1/9'>Compound 1</scene> is a fluorobiphenylcarboxylic acid. It occupies <scene name='Sandbox_reserved_394/Binding_site_1/ | + | <scene name='Sandbox_reserved_394/Compound_1/12'>Two fragments</scene> were found to have moderate affinity for Bcl-xl. <scene name='Sandbox_reserved_394/Compound_1/9'>Compound 1</scene> is a fluorobiphenylcarboxylic acid. It occupies <scene name='Sandbox_reserved_394/Binding_site_1/4'>binding site 1</scene> of Bcl-xl which consists of Phe 101, Tyr 105, Ala 108, Phe 109, Leu 136, Gly 142, Arg 143, and Ala 146. The fluorobiphenyl system of compound 1 is very hydrophobic and therefore, these residues form a <scene name='Sandbox_reserved_394/Compound_1/4'>"hydrophobic pocket"</scene> around the system. There is also one hydrophilic interaction involved in this complex. The <scene name='Sandbox_reserved_394/Compound_1/5'>carboxylic acid portion of compound 1 binds near Gly 142</scene> of Bcl-xl. This is not a strong interaction but is significant because it can be modified to form a much stronger bond. |
<scene name='Sandbox_reserved_394/Compound_1/3'>Compound 2</scene> is a napthalene-based alcohol which occupies <scene name='Sandbox_reserved_394/Binding_site_2/4'>binding site 2</scene>. This particular fragment also is involved with hydrophobic interactions with Bcl-xl, although they are not as strong as in the case of compound 1. This binding site includes Ala 97, Glu 100, Phe 101, Val 145, and Tyr 199. | <scene name='Sandbox_reserved_394/Compound_1/3'>Compound 2</scene> is a napthalene-based alcohol which occupies <scene name='Sandbox_reserved_394/Binding_site_2/4'>binding site 2</scene>. This particular fragment also is involved with hydrophobic interactions with Bcl-xl, although they are not as strong as in the case of compound 1. This binding site includes Ala 97, Glu 100, Phe 101, Val 145, and Tyr 199. | ||
| Line 56: | Line 56: | ||
! scope="col" width="5000px" | Modifying compound 3 to reduce HSA affinity | ! scope="col" width="5000px" | Modifying compound 3 to reduce HSA affinity | ||
|- | |- | ||
| - | | scope="col" width="5000px" | Compound 3 has high affinity for Bcl-xl but has an even higher affinity for HSA. For this reason, when HSA is present, compound 3 and similar ligands are more likely to bind to HSA thereby decreasing the amount that can bind with Bcl-xl. In order to decrease the affinity for HSA while maintaining affinity for Bcl-xl, SAR by NMR was used to compare compound 3 with a <scene name='Sandbox_reserved_394/Compound_3/1'>compound 4</scene> (thioethylamino-2,4-dimethylphenyl analogue), which also has high affinity for HSA. It was found that <scene name='Sandbox_reserved_394/Compound_3/ | + | | scope="col" width="5000px" | Compound 3 has high affinity for Bcl-xl but has an even higher affinity for HSA. For this reason, when HSA is present, compound 3 and similar ligands are more likely to bind to HSA thereby decreasing the amount that can bind with Bcl-xl. In order to decrease the affinity for HSA while maintaining affinity for Bcl-xl, SAR by NMR was used to compare compound 3 with a <scene name='Sandbox_reserved_394/Compound_3/1'>compound 4</scene> (thioethylamino-2,4-dimethylphenyl analogue), which also has high affinity for HSA. It was found that <scene name='Sandbox_reserved_394/Compound_3/3'>two hydrophobic portions</scene> of compound 4 had very strong hydrophobic interactions with HSA. Therefore, these portions in compound 3 were modified with polar substituents to decrease HSA affinity. To decrease hydrophobicity, the fluorobiphenyl system was substituted with a <scene name='Sandbox_reserved_394/Piperazine_ring/2'>piperazine ring</scene> and a <scene name='Sandbox_reserved_394/Abt-737/4'>2-dimethylaminoethyl group</scene> was added to the thioethylamino linkage group. |
|} | |} | ||
Current revision
Drug Design: Fragment-Based Drug Discovery
| |||||||||||
References
- ↑ 1.0 1.1 Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W. Discovering High-Affinity Ligands for Proteins: SAR by NMR. Science; Nov 29, 1996; 274, 5292; ProQuest Central pg. 1531.
- ↑ Oltersdorf T., Elmore S. W., Shoemaker A. R. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Vol 435|2 June 2005|doi:10.1038/nature03579
- ↑ Pandit D. LIGAND-BASED DRUG DESIGN: I. CONFORMATIONAL STUDIES OF GBR 12909 ANALOGS AS COCAINE ANTAGONISTS; II. 3D-QSAR STUDIES OF SALVINORIN A ANALOGS AS εΑΡΡΑ OPIOID AGONISTS. http://archives.njit.edu/vol01/etd/2000s/2007/njit-etd2007-051/njit-etd2007-051.pdf