Molecular Playground/Tic40
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == C-terminal NP-repeat domain of Tic40 == | + | ==C-terminal NP-repeat domain of Tic40== |
| - | + | <StructureSection load='2lnm' size='350' side='right' caption='C-terminal NP-repeat domain of Tic40(2) [[2lnm]]' scene=''> | |
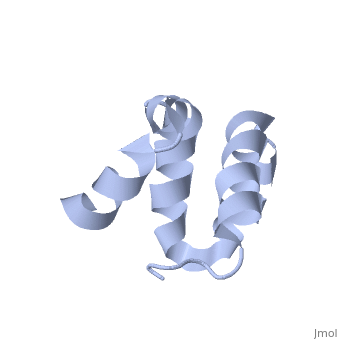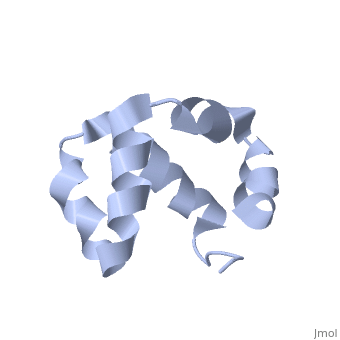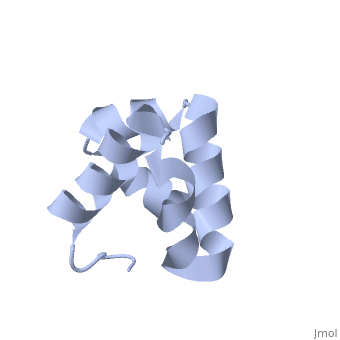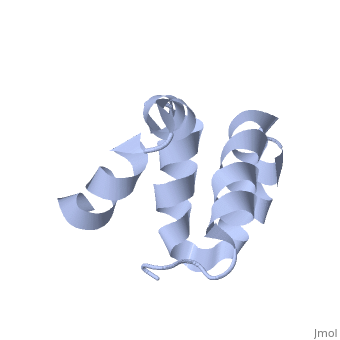
| - | + | <scene name='User:Mine_Canakci/Sandbox_1/Tic40-np_domain/1'> C-terminal NP-repeat domain of Tic40, a co-chaperone of plastid protein import </scene> | |
| - | + | Nuclear-encoded chloroplast proteins are synthesized in the cytosol with an N-terminal transit peptide. These proteins then traverse the chloroplast envelope membranes with the help of translocon machinery in the outer and inner envelope membranes called TOC and TIC, respectively (1). Tic40 is one of the essential components of TIC machinery. Tic40 together with Hsp93 involves in protein translocation to stroma. C-terminal Np-repeat domain of Tic40 interacts with Hsp93 and stimulates ATP hydrolysis (3). | |
| - | + | ||
| - | Nuclear-encoded chloroplast proteins are synthesized in the cytosol with an N-terminal transit peptide. These proteins then traverse the chloroplast envelope membranes with the help of translocon machinery in the outer and inner envelope membranes called TOC and TIC, respectively (1). Tic40 is one of the essential components of TIC machinery. | + | |
| + | </StructureSection> | ||
References: | References: | ||
| - | 1- Schleiff, E. and Becker, T. (2011) Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 12, | + | |
| + | 1- Schleiff, E. and Becker, T. (2011) Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 12, 48-59 | ||
2- Yi-Fen Kao,Yuan-Chao Lou,Yi-Hung Yeh,Chwan-Deng Hsiao,and Chinpan Chen. (2012) Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts. J Biochem 152(5): 443-451 | 2- Yi-Fen Kao,Yuan-Chao Lou,Yi-Hung Yeh,Chwan-Deng Hsiao,and Chinpan Chen. (2012) Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts. J Biochem 152(5): 443-451 | ||
| + | |||
| + | 3- Kovacheva, S., Bedard, J., Wardle, A., Patel, R., and Jarvis, P. (2007) Further in vivo studies on the role of the molecular chaperone, Hsp93, in plastid protein import. Plant J. 50, 364-379 | ||
Current revision
C-terminal NP-repeat domain of Tic40
| |||||||||||
References:
1- Schleiff, E. and Becker, T. (2011) Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 12, 48-59
2- Yi-Fen Kao,Yuan-Chao Lou,Yi-Hung Yeh,Chwan-Deng Hsiao,and Chinpan Chen. (2012) Solution structure of the C-terminal NP-repeat domain of Tic40, a co-chaperone during protein import into chloroplasts. J Biochem 152(5): 443-451
3- Kovacheva, S., Bedard, J., Wardle, A., Patel, R., and Jarvis, P. (2007) Further in vivo studies on the role of the molecular chaperone, Hsp93, in plastid protein import. Plant J. 50, 364-379