Sandbox UC 19
From Proteopedia
(Difference between revisions)
| (12 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== Leucine transporter LeuT in complex with sertraline == | == Leucine transporter LeuT in complex with sertraline == | ||
| + | |||
Recent high-resolution crystal structures of several transporters from protein families that were previously thought to be unrelated show common structural features indicating a large structural family representing transporters from all kingdoms of life. | Recent high-resolution crystal structures of several transporters from protein families that were previously thought to be unrelated show common structural features indicating a large structural family representing transporters from all kingdoms of life. | ||
| - | <StructureSection load='3gwu' size='350' side='right' caption='Leucine transporter LeuT in complex with sertraline (PDB entry [[3gwu]])' scene=''> | ||
| + | Content | ||
| + | <StructureSection load='3gwu' size='350' side='right' caption='Leucine transporter LeuT in complex with sertraline (PDB entry [[3gwu]])' scene=''> | ||
| + | =Introduction= | ||
'''Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures''' | '''Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures''' | ||
Sertraline and fluoxetine are selective serotonin re-uptake inhibitors (SSRIs) that are widely prescribed to treat depression <ref>PMID 23105100</ref>. They exert their effects by inhibiting the presynaptic plasma membrane serotonin transporter (SERT). All SSRIs possess halogen atoms at specific positions, which are key determinants for the drugs' specificity for SERT. For the SERT protein, however, the structural basis of its specificity for SSRIs is poorly understood. Here we report the crystal structures of LeuT, a bacterial SERT homolog, in complex with sertraline, R-fluoxetine or S-fluoxetine. The SSRI halogens all bind to exactly the same pocket within LeuT. Mutation at this halogen-binding pocket (HBP) in SERT markedly reduces the transporter's affinity for SSRIs but not for tricyclic antidepressants. Conversely, when the only nonconserved HBP residue in both norepinephrine and dopamine transporters is mutated into that found in SERT, their affinities for all the three SSRIs increase uniformly. Thus, the specificity of SERT for SSRIs is dependent largely on interaction of the drug halogens with the protein's HBP. | Sertraline and fluoxetine are selective serotonin re-uptake inhibitors (SSRIs) that are widely prescribed to treat depression <ref>PMID 23105100</ref>. They exert their effects by inhibiting the presynaptic plasma membrane serotonin transporter (SERT). All SSRIs possess halogen atoms at specific positions, which are key determinants for the drugs' specificity for SERT. For the SERT protein, however, the structural basis of its specificity for SSRIs is poorly understood. Here we report the crystal structures of LeuT, a bacterial SERT homolog, in complex with sertraline, R-fluoxetine or S-fluoxetine. The SSRI halogens all bind to exactly the same pocket within LeuT. Mutation at this halogen-binding pocket (HBP) in SERT markedly reduces the transporter's affinity for SSRIs but not for tricyclic antidepressants. Conversely, when the only nonconserved HBP residue in both norepinephrine and dopamine transporters is mutated into that found in SERT, their affinities for all the three SSRIs increase uniformly. Thus, the specificity of SERT for SSRIs is dependent largely on interaction of the drug halogens with the protein's HBP. | ||
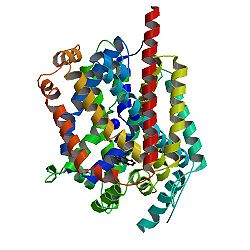
| - | <Structure load='3gwu' size='250' frame='true' align=' | + | <Structure load='3gwu' size='250' frame='true' align='left' caption=''''3D LeuT Structure'''' scene='' /> |
[[Image:3gwu Static.jpg|250px|center]] | [[Image:3gwu Static.jpg|250px|center]] | ||
| Line 14: | Line 17: | ||
{{Template:ColorKey_Amino2CarboxyRainbow}} | {{Template:ColorKey_Amino2CarboxyRainbow}} | ||
| - | <scene name='Sandbox_UC_19/Leut2/1'>2nd Structure</scene> | ||
| - | {{Template:ColorKey_Helix}} | ||
| + | ==Mechanism== | ||
| + | |||
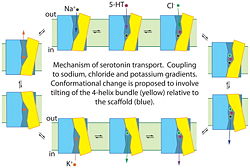
| + | [[Image:Mecanismo.jpg|250px|center]] | ||
| + | '''Proposed mechanism for serotonin transport by SERT'''. Binding of serotonin (5-HT+), Na+ and Cl− to the transporter from the cell exterior allows a conformational change, shown here as the tilting of a 4-helix bundle (yellow), that closes the extracellular substrate permeation pathway and opens a cytoplasmic pathway. After dissociation of Na+, Cl− and 5-HT+ to the cytoplasm, the transporter binds a K+ ion (or a proton, see (96)) to allow the reverse conformational change, leading to extracellular K+ dissociation and another cycle of transport. | ||
| + | |||
| + | asdasdsdfsadfsdfasfsddf [[transporter|SERT]] sadasdasdasdasd sdfsd wfsdfsd wfsdfsdf | ||
| Line 22: | Line 29: | ||
<references/> | <references/> | ||
| - | [[fr:leve | ||
Current revision
Leucine transporter LeuT in complex with sertraline
Recent high-resolution crystal structures of several transporters from protein families that were previously thought to be unrelated show common structural features indicating a large structural family representing transporters from all kingdoms of life.
Content
| |||||||||||