We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
2bo5
From Proteopedia
(Difference between revisions)
| (13 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:2bo5.gif|left|200px]]<br /><applet load="2bo5" size="350" color="white" frame="true" align="right" spinBox="true" | ||
| - | caption="2bo5" /> | ||
| - | '''BOVINE OLIGOMYCIN SENSITIVITY CONFERRAL PROTEIN N-TERMINAL DOMAIN'''<br /> | ||
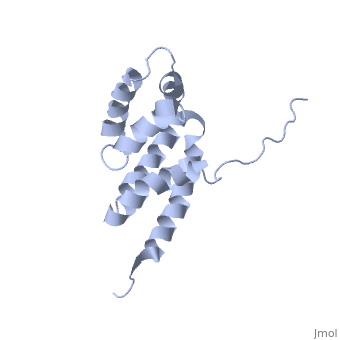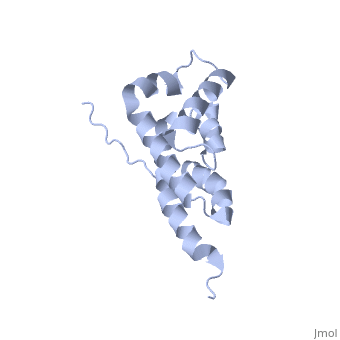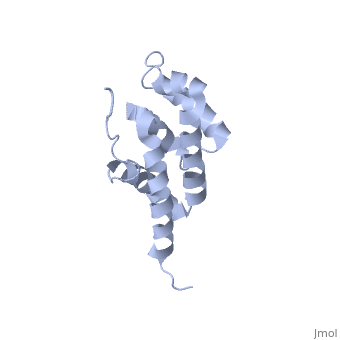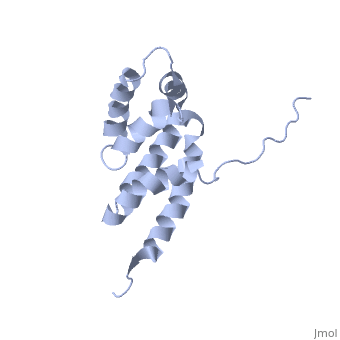
| - | == | + | ==Bovine oligomycin sensitivity conferral protein N-terminal domain== |
| + | <StructureSection load='2bo5' size='340' side='right'caption='[[2bo5]]' scene=''> | ||
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[2bo5]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Bos_taurus Bos taurus]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2BO5 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2BO5 FirstGlance]. <br> | ||
| + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">Solution NMR</td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2bo5 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2bo5 OCA], [https://pdbe.org/2bo5 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2bo5 RCSB], [https://www.ebi.ac.uk/pdbsum/2bo5 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2bo5 ProSAT]</span></td></tr> | ||
| + | </table> | ||
| + | == Function == | ||
| + | [https://www.uniprot.org/uniprot/ATPO_BOVIN ATPO_BOVIN] Mitochondrial membrane ATP synthase (F(1)F(0) ATP synthase or Complex V) produces ATP from ADP in the presence of a proton gradient across the membrane which is generated by electron transport complexes of the respiratory chain. F-type ATPases consist of two structural domains, F(1) - containing the extramembraneous catalytic core and F(0) - containing the membrane proton channel, linked together by a central stalk and a peripheral stalk. During catalysis, ATP synthesis in the catalytic domain of F(1) is coupled via a rotary mechanism of the central stalk subunits to proton translocation. Part of the complex F(0) domain and the peripheric stalk, which acts as a stator to hold the catalytic alpha(3)beta(3) subcomplex and subunit a/ATP6 static relative to the rotary elements. | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/bo/2bo5_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=2bo5 ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| + | <div style="background-color:#fffaf0;"> | ||
| + | == Publication Abstract from PubMed == | ||
The peripheral stalk of ATP synthase holds the alpha3beta3 catalytic subcomplex stationary against the torque of the rotating central stalk. In bovine mitochondria, the N-terminal domain of the oligomycin sensitivity conferral protein (OSCP-NT; residues 1-120) anchors one end of the peripheral stalk to the N-terminal tails of one or more alpha-subunits of the F1 subcomplex. Here we present the solution structure of OSCP-NT and an NMR titration study of its interaction with peptides representing N-terminal tails of F1 alpha-subunits. The structure comprises a bundle of six alpha-helices, and its interaction site contains adjoining hydrophobic surfaces of helices 1 and 5; residues in the region 1-8 of the alpha-subunit are essential for the interaction. The OSCP-NT is similar to the N-terminal domain of the delta-subunit from Escherichia coli ATP synthase (delta-NT), except that their surface charges differ (basic and acidic, respectively). As the charges of the adjacent crown regions in their alpha3beta3 complexes are similar, the OSCP-NT and delta-NT probably do not contact the crowns extensively. The N-terminal tails of alpha-subunit tails are probably alpha-helical, and so this interface, which is essential for the rotary mechanism of the enzyme, appears to consist of helix-helix interactions. | The peripheral stalk of ATP synthase holds the alpha3beta3 catalytic subcomplex stationary against the torque of the rotating central stalk. In bovine mitochondria, the N-terminal domain of the oligomycin sensitivity conferral protein (OSCP-NT; residues 1-120) anchors one end of the peripheral stalk to the N-terminal tails of one or more alpha-subunits of the F1 subcomplex. Here we present the solution structure of OSCP-NT and an NMR titration study of its interaction with peptides representing N-terminal tails of F1 alpha-subunits. The structure comprises a bundle of six alpha-helices, and its interaction site contains adjoining hydrophobic surfaces of helices 1 and 5; residues in the region 1-8 of the alpha-subunit are essential for the interaction. The OSCP-NT is similar to the N-terminal domain of the delta-subunit from Escherichia coli ATP synthase (delta-NT), except that their surface charges differ (basic and acidic, respectively). As the charges of the adjacent crown regions in their alpha3beta3 complexes are similar, the OSCP-NT and delta-NT probably do not contact the crowns extensively. The N-terminal tails of alpha-subunit tails are probably alpha-helical, and so this interface, which is essential for the rotary mechanism of the enzyme, appears to consist of helix-helix interactions. | ||
| - | + | Structure of the F1-binding domain of the stator of bovine F1Fo-ATPase and how it binds an alpha-subunit.,Carbajo RJ, Kellas FA, Runswick MJ, Montgomery MG, Walker JE, Neuhaus D J Mol Biol. 2005 Aug 26;351(4):824-38. PMID:16045926<ref>PMID:16045926</ref> | |
| - | + | ||
| - | + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |
| - | + | </div> | |
| + | <div class="pdbe-citations 2bo5" style="background-color:#fffaf0;"></div> | ||
| + | == References == | ||
| + | <references/> | ||
| + | __TOC__ | ||
| + | </StructureSection> | ||
[[Category: Bos taurus]] | [[Category: Bos taurus]] | ||
| - | [[Category: | + | [[Category: Large Structures]] |
| - | + | [[Category: Carbajo RJ]] | |
| - | [[Category: Carbajo | + | [[Category: Kellas FA]] |
| - | [[Category: Kellas | + | [[Category: Montgomery MG]] |
| - | [[Category: Montgomery | + | [[Category: Neuhaus D]] |
| - | [[Category: Neuhaus | + | [[Category: Runswick MJ]] |
| - | [[Category: Runswick | + | [[Category: Walker JE]] |
| - | [[Category: Walker | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
Bovine oligomycin sensitivity conferral protein N-terminal domain
| |||||||||||