We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
F-actin
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='2zwh' size='350' side='right' scene='' caption='Rabbit α-actin complex with ADP and Ca+2 ion (PDB code [[2zwh]])'> | |
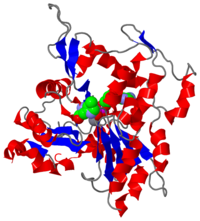
[[Image:2zwh.png|left|200px|thumb|Crystal Structure of F-actin, [[2zwh]]]]'''Filamentous actin''' ('''F-actin''') units are also referred to as [http://en.wikipedia.org/wiki/microfilament microfilaments] <ref> Microfilaments - Wikipedia, the free encyclopedia. http://en.wikipedia.org/wiki/Microfilament. Date accessed: March 16th, 2010. </ref> and are highly conserved, proteinous components found near ubiquitously in eukaryotic cytoskeletons. F-actin and other [[actin]] proteins generally have structural roles in cells. | [[Image:2zwh.png|left|200px|thumb|Crystal Structure of F-actin, [[2zwh]]]]'''Filamentous actin''' ('''F-actin''') units are also referred to as [http://en.wikipedia.org/wiki/microfilament microfilaments] <ref> Microfilaments - Wikipedia, the free encyclopedia. http://en.wikipedia.org/wiki/Microfilament. Date accessed: March 16th, 2010. </ref> and are highly conserved, proteinous components found near ubiquitously in eukaryotic cytoskeletons. F-actin and other [[actin]] proteins generally have structural roles in cells. | ||
<br/> | <br/> | ||
| Line 9: | Line 9: | ||
== Assembly == | == Assembly == | ||
| - | < | + | <scene name='Sandbox_154/1j6z_black_true/2'>Globular Actin (G-actin)</scene> ([http://www.rcsb.org/pdb/explore/explore.do?structureId=1J6Z 1J6Z]). |
| + | |||
'''G-actin''' is the free monomeric form of actin which polymerizes to F-actin. The structures of globular and filamentous actin are distinct from one another in numerous ways, despite the fact that G-actin comprises F-actin. When the monomeric actin becomes polymerized into F-actin, the unit becomes flattened. Also, F-actin possesses an ATPase function which is minimal in G-actin. The domains and active site are the same in terms of constituent components and will be discussed later in terms of the F-actin monomer. | '''G-actin''' is the free monomeric form of actin which polymerizes to F-actin. The structures of globular and filamentous actin are distinct from one another in numerous ways, despite the fact that G-actin comprises F-actin. When the monomeric actin becomes polymerized into F-actin, the unit becomes flattened. Also, F-actin possesses an ATPase function which is minimal in G-actin. The domains and active site are the same in terms of constituent components and will be discussed later in terms of the F-actin monomer. | ||
| Line 25: | Line 26: | ||
=== F-actin Monomer and Polymer === | === F-actin Monomer and Polymer === | ||
| - | < | + | <scene name='Sandbox_154/2zwh_regions/2'>Filamentous Actin Unit (F-actin)</scene> ([[2zwh]]) |
==== Monomer ==== | ==== Monomer ==== | ||
| Line 44: | Line 45: | ||
==== Domains ==== | ==== Domains ==== | ||
| - | < | + | <scene name='Sandbox_154/2zwh_black_domains/1'>Filamentous Actin (F-actin)</scene> ([[2zwh]]) |
| + | |||
The structure of a single unit of F-actin arises from one polypeptide chain with two domains. The nucleotide binding cleft, site of ATP hydrolysis, can be observed between the two domains. Movement of the domains allows for the open and closed F-actin conformations. | The structure of a single unit of F-actin arises from one polypeptide chain with two domains. The nucleotide binding cleft, site of ATP hydrolysis, can be observed between the two domains. Movement of the domains allows for the open and closed F-actin conformations. | ||
Domain movement is made possible by rotation about the <scene name='Sandbox_154/2zwh_helix_domains_2/1'> peptide bonds of residues 141-142 and 335-336</scene>, shown in purple. According to Oda et al., during the transition from G- to F- actin, Domain 2 is believed to tilt 20° and fit itself with Domain 1, thus giving a flatter conformation than the free G-actin. It is not certain whether this flattening occurs before or after ATP hydrolysis<ref name="oda" />. Holmes<ref name="Holmes2">PMID:2395461</ref> provides a simplified image of this domain movement and flattening[http://www.nature.com/nature/journal/v457/n7228/fig_tab/457389a_F2.html]. | Domain movement is made possible by rotation about the <scene name='Sandbox_154/2zwh_helix_domains_2/1'> peptide bonds of residues 141-142 and 335-336</scene>, shown in purple. According to Oda et al., during the transition from G- to F- actin, Domain 2 is believed to tilt 20° and fit itself with Domain 1, thus giving a flatter conformation than the free G-actin. It is not certain whether this flattening occurs before or after ATP hydrolysis<ref name="oda" />. Holmes<ref name="Holmes2">PMID:2395461</ref> provides a simplified image of this domain movement and flattening[http://www.nature.com/nature/journal/v457/n7228/fig_tab/457389a_F2.html]. | ||
| Line 69: | Line 71: | ||
==== [http://en.wikipedia.org/wiki/Actin#Actomyosin_filaments Actin-Myosin] ==== | ==== [http://en.wikipedia.org/wiki/Actin#Actomyosin_filaments Actin-Myosin] ==== | ||
The relatively flatter shape of F-actin as compared to G-actin allows myosin to preferentially bind F-actin over G-actin. This means that F-actin, not G-actin, is the functional form of actin. It composes a large part of the thin filaments in conjunction with mysoin to give muscle contractions<ref name="Holmes2"/><ref name="Holmes3>PMID:14508495</ref>. The structure of F-actin gives it large resistance to extensive forces, such as those experienced in muscle contraction<ref name="Mitchinson"/>. | The relatively flatter shape of F-actin as compared to G-actin allows myosin to preferentially bind F-actin over G-actin. This means that F-actin, not G-actin, is the functional form of actin. It composes a large part of the thin filaments in conjunction with mysoin to give muscle contractions<ref name="Holmes2"/><ref name="Holmes3>PMID:14508495</ref>. The structure of F-actin gives it large resistance to extensive forces, such as those experienced in muscle contraction<ref name="Mitchinson"/>. | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
==3D structures of actin== | ==3D structures of actin== | ||
Current revision
| |||||||||||
3D structures of actin
References
- ↑ Microfilaments - Wikipedia, the free encyclopedia. http://en.wikipedia.org/wiki/Microfilament. Date accessed: March 16th, 2010.
- ↑ 2.0 2.1 2.2 2.3 2.4 Graceffa P, Dominguez R. Crystal structure of monomeric actin in the ATP state. Structural basis of nucleotide-dependent actin dynamics. J Biol Chem. 2003 Sep 5;278(36):34172-80. Epub 2003 Jun 17. PMID:12813032 doi:10.1074/jbc.M303689200
- ↑ 3.0 3.1 Holmes KC. Structural biology: actin in a twist. Nature. 2009 Jan 22;457(7228):389-90. PMID:19158779 doi:10.1038/457389a
- ↑ 4.0 4.1 4.2 4.3 Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44-9. PMID:2395461 doi:http://dx.doi.org/10.1038/347044a0
- ↑ 5.0 5.1 5.2 Otterbein LR, Graceffa P, Dominguez R. The crystal structure of uncomplexed actin in the ADP state. Science. 2001 Jul 27;293(5530):708-11. PMID:11474115 doi:10.1126/science.1059700
- ↑ 6.0 6.1 6.2 Pfaendtner J, Branduardi D, Parrinello M, Pollard TD, Voth GA. Nucleotide-dependent conformational states of actin. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12723-8. Epub 2009 Jul 20. PMID:19620726
- ↑ 7.0 7.1 7.2 Mitchison TJ. Compare and contrast actin filaments and microtubules. Mol Biol Cell. 1992 Dec;3(12):1309-15. PMID:1493331
- ↑ 8.0 8.1 Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci. 2000 Jan;25(1):19-23. PMID:10637608
- ↑ Carlier MF, Pantaloni D. Direct evidence for ADP-Pi-F-actin as the major intermediate in ATP-actin polymerization. Rate of dissociation of Pi from actin filaments. Biochemistry. 1986 Dec 2;25(24):7789-92. PMID:3801442
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 Oda T, Iwasa M, Aihara T, Maeda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009 Jan 22;457(7228):441-5. PMID:19158791 doi:10.1038/nature07685
- ↑ Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990 Sep 6;347(6288):44-9. PMID:2395461 doi:http://dx.doi.org/10.1038/347044a0
- ↑ Oztug Durer ZA, Diraviyam K, Sept D, Kudryashov DS, Reisler E. F-actin structure destabilization and DNase I binding loop: fluctuations mutational cross-linking and electron microscopy analysis of loop states and effects on F-actin. J Mol Biol. 2010 Jan 22;395(3):544-57. Epub 2009 Nov 6. PMID:19900461 doi:10.1016/j.jmb.2009.11.001
- ↑ Oztug Durer ZA, Diraviyam K, Sept D, Kudryashov DS, Reisler E. F-actin structure destabilization and DNase I binding loop: fluctuations mutational cross-linking and electron microscopy analysis of loop states and effects on F-actin. J Mol Biol. 2010 Jan 22;395(3):544-57. Epub 2009 Nov 6. PMID:19900461 doi:10.1016/j.jmb.2009.11.001
- ↑ Dalhaimer P, Pollard TD, Nolen BJ. Nucleotide-mediated conformational changes of monomeric actin and Arp3 studied by molecular dynamics simulations. J Mol Biol. 2008 Feb 8;376(1):166-83. Epub 2007 Nov 28. PMID:18155236 doi:10.1016/j.jmb.2007.11.068
- ↑ Pfaendtner J, Lyman E, Pollard TD, Voth GA. Structure and dynamics of the actin filament. J Mol Biol. 2010 Feb 19;396(2):252-63. Epub 2009 Nov 18. PMID:19931282 doi:10.1016/j.jmb.2009.11.034
- ↑ Stricker J, Falzone T, Gardel ML. Mechanics of the F-actin cytoskeleton. J Biomech. 2010 Jan 5;43(1):9-14. Epub 2009 Nov 13. PMID:19913792 doi:10.1016/j.jbiomech.2009.09.003
- ↑ Holmes KC, Angert I, Kull FJ, Jahn W, Schroder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003 Sep 25;425(6956):423-7. PMID:14508495 doi:10.1038/nature02005
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Melissa Chow, Alexander Berchansky